Lymphovascular invasion in colorectal cancers: can we predict it preoperatively?
Article information
Abstract
Purpose
This study aimed to investigate preoperative predictors of lymphovascular invasion (LVI), which is a poor prognostic factor usually detected postoperatively in patients with colorectal cancer.
Methods
Results for all patients operated on for colorectal cancer between January 1, 2006, and December 31, 2021, were retrospectively analyzed. Potential preoperative factors and postoperative pathology results were recorded. The patients were categorized as those with LVI and those without LVI. Potential factors that may be associated with LVI were compared between the 2 groups.
Results
The study included 335 patients. The incidence of LVI was 3.11 times higher in patients with ascending colon tumors (odds ratio [OR], 3.11; 95% confidence interval [CI], 1.34–7.23; P=0.008) and 4.28 times higher in those with metastatic tumors (OR, 4.28; 95% CI, 2.18–8.39; P<0.001). Diabetes mellitus was inversely related to LVI in colorectal cancer patients; specifically, LVI was 56% less common in colorectal cancer patients with diabetes mellitus, irrespective of its duration (OR, 0.44; 95% CI, 0.25–0.76; P<0.001).
Conclusion
The presence of preoperative LVI in colorectal cancer patients is difficult to predict. In particular, the effect of chronic factors accompanied by microvascular pathologies on LVI is still unclear. Advances in the neoadjuvant treatment of colorectal cancer patients, who are becoming more widespread every day, will encourage the investigation of different methods of preoperatively predicting LVI as a poor prognostic factor in these patients.
INTRODUCTION
Colorectal cancer (CRC) is among the most common types of cancer. Approximately 1.5 million patients are newly diagnosed worldwide every year; this number will soon exceed 2 million [1]. CRC treatment generally takes a multidisciplinary approach, and surgery, chemotherapy, and radiotherapy are applied depending on the stage of the disease. In recent years, the number of studies recommending neoadjuvant chemotherapy (NCT) in CRC treatment, especially in the locally advanced stage, has been increasing [2–4]. Many studies investigating the effects of neoadjuvant therapy on survival and relapse have reported positive results, and CRC patients may benefit from this treatment when they have accompanying poor prognostic factors [3, 5]. Therefore, it has become crucial to determine poor prognostic factors in CRC patients [2, 4]. Preoperative radiological and laboratory methods can determine the following factors: distant metastasis, invasion of surrounding organs, high carcinoembryonic antigen (CEA) value, and regional metastatic lymph node involvement. However, the following parameters are impossible to determine before surgery: definitive T category, definitive metastatic lymph node count, perineural invasion, and lymphovascular invasion (LVI). LVI is a prognostic factor independent of and worse than lymph node involvement, which can cause early metastasis as tumor cells cross the endothelial barrier in CRC patients [6–10]. In fact, LVI is a poor prognostic factor in CRC patients with negative lymph nodes [11]. Although the exact mechanism and etiological factors of LVI in tumor tissues are still unclear, studies are ongoing on this subject [9, 12]. NCT is thought to be effective for preventing the development of local micrometastases that develop early in this patient group [13]. Therefore, several studies have attempted to predict the presence of LVI preoperatively in CRC patients [14–16]. However, these studies have provided limited information on the preoperative prediction of LVI due to including a small number of patients and a limited range of examinations, such as only radiological evaluation and blood test results. The effects on LVI of chronic factors such as diabetes mellitus (DM), hypertension (HT), and smoking, which cause deterioration of microvascular permeability, are not sufficiently known. For this reason, there is a need for studies involving many preoperative factors, including chronic diseases and behaviors. In this study, we aimed to examine whether various parameters are associated with LVI in CRC patients undergoing surgery to identify whether any of those parameters are useful for predicting LVI preoperatively.
METHODS
Ethics statement
This study was approved by the Ethics Committee of the Istanbul Yeni Yüzyıl University (No. 2022/07-884). Written informed consent were obtained from the patients.
Study design and patients
The results of all patients of both sexes operated on for CRC between January 1, 2006, and December 31, 2021, were retrospectively examined. All patients who had been diagnosed histologically with CRC were included in this study. Patients whose treatment was not completed at our center, who received neoadjuvant chemotherapy, and whose surgery occurred under emergency conditions were excluded from the study. The study analyzed the following information: age, sex, additional chronic diseases and their duration, complete blood count results, smoking, alcohol consumption status and its duration, American Society of Anesthesiologists (ASA) physical status, tumor location, preoperative positron emission tomography–computed tomography (PET-CT) results for eradication, the tumor’s maximum standardized uptake value (SUVmax), its radiologically determined size, its estimated volume, and metastasis status. In addition, the patients’ postoperative pathology results were examined. Detection of LVI was routinely determined by 2 pathologists using hematoxylin-eosin and elastin staining. The patients were categorized as those with LVI and those without LVI.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess whether the variables followed a normal distribution. Continuous variables were presented as median (range, minimum to maximum). Categorical variables were reported as numbers and percentages. According to the normality test results, the Mann-Whitney U-test was used to compare the 2 groups. The Pearson chi-square test, Fisher exact test, and Fisher-Freeman-Halton test were used for comparing categorical variables. Multiple logistic regression analysis was performed to determine the risk factors affecting the incidence of LVI. Variables were included in the multiple logistic regression model by using the forward likelihood ratio method. The significant variables in the model became independent variables. The multiple logistic regression models were statistically significant (P<0.001). IBM SPSS ver. 21.0 (IBM Corp) was used for statistical analysis, and a P-value <0.05 was set as the threshold for statistical significance.
RESULTS
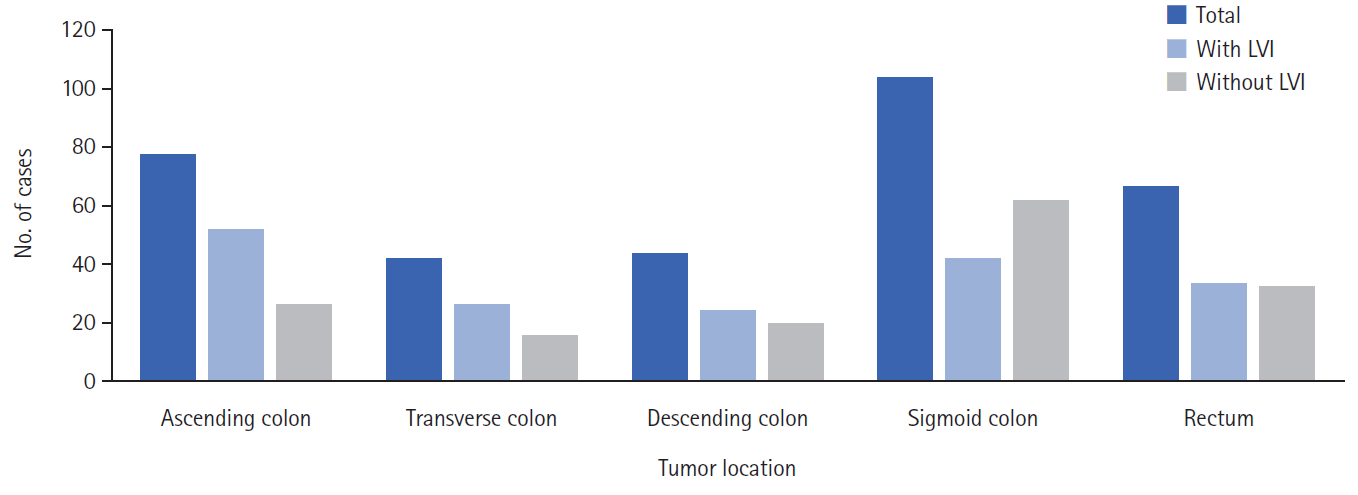
A total of 335 patients, 146 women (43.6%) and 189 men (56.4%), were included in the study. All patients had adenocarcinoma, and 63 patients (18.8%) had mucinous components. Preoperative metastasis was present in 57 patients (17.0%); 34 patients (10.1%) had preoperative radiotherapy due to rectal cancer. Regarding tumor type, 78 patients (23.3%) had ascending tumors, 42 patients (12.5%) had transverse tumors, 44 patients (13.1%) had descending tumors, 104 patients (31.0%) had sigmoid colon tumors, and 67 patients (20.0%) had rectal tumors. LVI was present in 178 patients (53.1%) (Fig. 1). Other poor prognostic factors in CRC were significantly more common in patients with LVI (Table 1).
The univariate analysis between the groups found several factors to be more prevalent in patients with LVI, including ascending colon tumor location (P=0.007), the presence of preoperative metastasis (P<0.001), a high ASA physical status (P=0.015), low hemoglobin value (P=0.008), low lymphocyte value (P=0.043), and radiologically measured tumor thickness (P=0.020). In contrast, DM was significantly less common in patients with LVI (P=0.003) (Table 2).
The multivariate analysis, performed by including the significant factors from the univariate analysis, found that the incidence of LVI was 3.11 times higher in patients with ascending colon tumors (P=0.008; 95% confidence interval [CI], 1.34–7.23) and 4.28 times higher in those with metastatic tumors (OR, 4.28; 95% CI, 2.18–8.39; P<0.001). DM was inversely related to LVI in colorectal cancer patients; specifically, LVI was 56% less common in colorectal cancer patients with DM, irrespective of its duration (OR, 0.44; 95% CI, 0.25–0.76; P<0.001) (Table 3).
DISCUSSION
This study aimed to identify possible preoperative factors likely to be associated with LVI in CRC patients. Studies have indicated that LVI is present in 5.2% to 89.5% of patients, depending on the CRC stage [17–21]. In our study, this proportion was 53.13%, which is consistent with the literature.
LVI was previously considered not to be a significant factor in CRC patients [22, 23]. Later, perceptions changed, and LVI came to be viewed as a stage-independent poor prognostic factor for CRC patients. In fact, in a study examining T3N0 patients with negative lymph nodes [24], LVI was a critical negative prognostic factor after adjuvant treatment and considering it during the treatment process was suggested. Lee et al. [25] stated that LVI had a poor prognostic effect in rectal cancer (RC) patients receiving neoadjuvant chemoradiotherapy and emphasized that treatments might be more effective if LVI status were known in advance. Zhang et al. [16] reported similar results. Wang et al. [7] reported that better treatment options could be determined for CRC patients by developing a new survival nomogram containing LVI. Therefore, it is recommended to monitor treatment particularly carefully in CRC patients with LVI and follow-up frequently with them. Huh et al. [26] proposed a new histological rating to be used in the follow-up of CRC patients, which considers the presence of LVI.
Although many studies have shown that DM is associated with poor prognosis in CRC patients, as in all cancers, its effect on LVI remains a matter of debate [27–29]. Little information in the literature provides an idea of how LVI is affected in CRC patients with DM. Some studies have reported that DM did not affect LVI in CRC patients [30–32] or that LVI was more common in CRC patients with DM [28]. In contrast, in our study the prevalence of DM was significantly lower in patients with LVI. LVI was detected in 78 patients (38.6%) with DM and 257 patients (57.6%) without DM. The results of our study differ from those previously reported in literature, although relatively few studies have dealt with this topic. Hyperglycemia in DM patients causes endothelial and wall damage in microvascular structures by using different complex biochemical pathways, leading to dysfunction [33, 34]. Studies have shown that this process starts during the prediabetes period [35]. Increased permeability in microvascular structures damages tissues due to perivascular inflammation and fibrosis [32, 35, 36]. As a result, the microvascular and perivascular pathological condition may reduce the migration of tumor cells and lower the incidence of LVI in this patient group. Adverse effects of using metformin on tumors are known in patients with CRC with type 2 DM [37]. It is thought that metformin use may also have an effect on LVI. In our study, however, LVI was not found to be associated with the type of diabetes (P=0.301) or the duration of diabetes (P=0.061). As seen from our results, the behavior of CRC cells in the case of impaired microvascular permeability, in which complex mechanisms related to DM play a role, warrants further investigation [28, 33]. Except for DM, other chronic conditions or behavior (HT, coronary artery disease, chronic obstructive pulmonary disease, chronic renal failure, smoking, and alcohol use) were not associated with LVI.
Although LVI is a significant prognostic factor, it is usually detected postoperatively. In their respective studies, Janjan et al. [38] and Du et al. [39] reported that preoperative radiotherapy did not affect LVI. For this reason, we included RC patients who received preoperative radiotherapy in our study. Some studies conducted to determine the presence of preoperative LVI have been reported in the literature. In their study examining RC, according to Ge et al. [15], the tumor volume obtained as a result of computed tomography may help determine LVI. Zhang et al. [16] suggested using a new multimodal radiomics model to determine the presence of LVI preoperatively in patients with RC. Kim et al. [40] tried to determine LVI in RC patients using preoperative pelvic magnetic resonance imaging. They detected LVI with 68.2% sensitivity and 93.2% specificity using perivascular infiltrative signals. Generally, such studies are related to RC, and there is no comprehensive literature on colon cancer. In our study, no statistically significant differences were found according to tumor volume measured by preoperative radiological methods or SUVmax measured by PET-CT.
In many studies, LVI rates are high in metastatic CRC patients. Studies have shown that as a poor prognostic factor, LVI is associated with relapse and distant metastasis [27, 40, 41]. This resulted from the transfer of tumor cells to circulatory system due to LVI. In our study, LVI was present at a higher rate in CRC patients with metastatic disease, which is compatible with the literature.
The prevalence of LVI varies according to the tumor location in CRC patients, and studies have generally indicated the right and left sides of the colon and the rectum region. In a retrospective cohort study involving 158,777 patients with T1 and T2 tumors, Al-Sukhni et al. [20] detected LVI in the appendix (25.1%), colon (28.1%), rectosigmoid (27.4%), and tumors originating from the rectum (18.1%). Zhong et al. [8] found no significant relationship between LVI and tumor site (right colon, left colon, and rectum) in their study involving stage III CRC patients. Similarly, in other studies involving different-stage CRC patients, no relationship was observed between the tumor site and LVI [42–45]. In our study, 52 patients (29.2%) with LVI had a tumor in the ascending colon, 26 patients (14.6%) had a tumor in the transverse colon, 24 patients (13.5%) had a tumor in the descending colon, 42 patients (23.6%) had a tumor in the sigmoid colon, and 34 patients (19.1%) had a tumor located in the rectum. We found that LVI was significantly more common in CRC located in the ascending colon than in the other colon segments (OR, 3.11; 95% CI, 1.34–7.23; P=0.008). Two factors may be relevant for explaining this finding: first, tumors are detected in the ascending colon more frequently in the advanced T category; and second, a high T category is associated with LVI [7, 27, 46]. In fact, 24 of 52 patients (46.2%) with T4 tumors had a mass located in the ascending colon in our study.
The major limitation of this study is that it was single-center and retrospective. In retrospective studies, errors may occur in the data because of recall bias or incorrect recording. A second significant limitation is that data on tumor markers and other factors, such as glycated hemoglobin, were not available for predicting LVI.
In conclusion, the preoperative LVI status in CRC patients has still not been studied adequately. The effects on LVI of chronic diseases, such as DM, especially those accompanied by microvascular pathologies, remain unclear. Advances in the neoadjuvant treatment of CRC patients, who are becoming more widespread every day, will encourage the investigation of different methods of preoperatively predicting LVI as a poor prognostic factor in these patients.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: EZ; Data curation: EZ, SÇ, Formal analysis: SÇ; Investigation: EZ, NT; Methodology: EZ, MÇ; Project administration: MÇ, SÇ; Visualization: EZ; Writing–original draft: EZ, SÇ, NT; Writing–review & editing: SÇ, MÇ. All authors read and approved the final manuscript.