Laser hemorrhoidoplasty versus conventional hemorrhoidectomy for grade II/III hemorrhoids: a systematic review and meta-analysis
Article information
Abstract
Purpose
This study compared the short- and long-term clinical outcomes of laser hemorrhoidoplasty (LH) vs. conventional hemorrhoidectomy (CH) in patients with grade II/III hemorrhoids.
Methods
PubMed/Medline and the Cochrane Library were searched for randomized and nonrandomized studies comparing LH against CH in grade II/III hemorrhoids. The primary outcomes included postoperative use of analgesia, postoperative morbidity (bleeding, urinary retention, pain, thrombosis), and time of return to work/daily activities.
Results
Nine studies totaling 661 patients (LH, 336 and CH, 325) were included. The LH group had shorter operative time (P<0.001) and less intraoperative blood loss (P<0.001). Postoperative pain was lower in the LH group, with lower postoperative day 1 (mean difference [MD], –2.09; 95% confidence interval [CI], –3.44 to –0.75; P=0.002) and postoperative day 7 (MD, –3.94; 95% CI, –6.36 to –1.52; P=0.001) visual analogue scores and use of analgesia (risk ratio [RR], 0.59; 95% CI, 0.42–0.81; P=0.001). The risk of postoperative bleeding was also lower in the LH group (RR, 0.18; 95% CI, 0.12– 0.28; P<0.001), with a quicker return to work or daily activities (P=0.002). The 12-month risks of bleeding (P>0.999) and prolapse (P=0.240), and the likelihood of complete resolution at 12 months, were similar (P=0.240).
Conclusion
LH offers more favorable short-term clinical outcomes than CH, with reduced morbidity and pain and earlier return to work or daily activities. Medium-term symptom recurrence at 12 months was similar. Our results should be verified in future well-designed trials with larger samples.
INTRODUCTION
Symptomatic hemorrhoids remain a common disease, with a global incidence of about 4% [1, 2]. While conservative or medical therapy is the first-line treatment in many instances, refractory symptoms of rectal bleeding, pain, itching, and tissue prolapse often necessitate procedural interventions, including surgery. While numerous surgical options have been described, none has yet proven to be the gold standard [3-6].
Laser therapy has been widely employed in medicine and surgery, proving comparable or even superior to traditional surgical approaches for conditions including liver cancer [7], prostate cancer [8], and various gynecological conditions [9]. The ablative effect of lasers is dependent on the irradiance (power density) and duration of application [10].
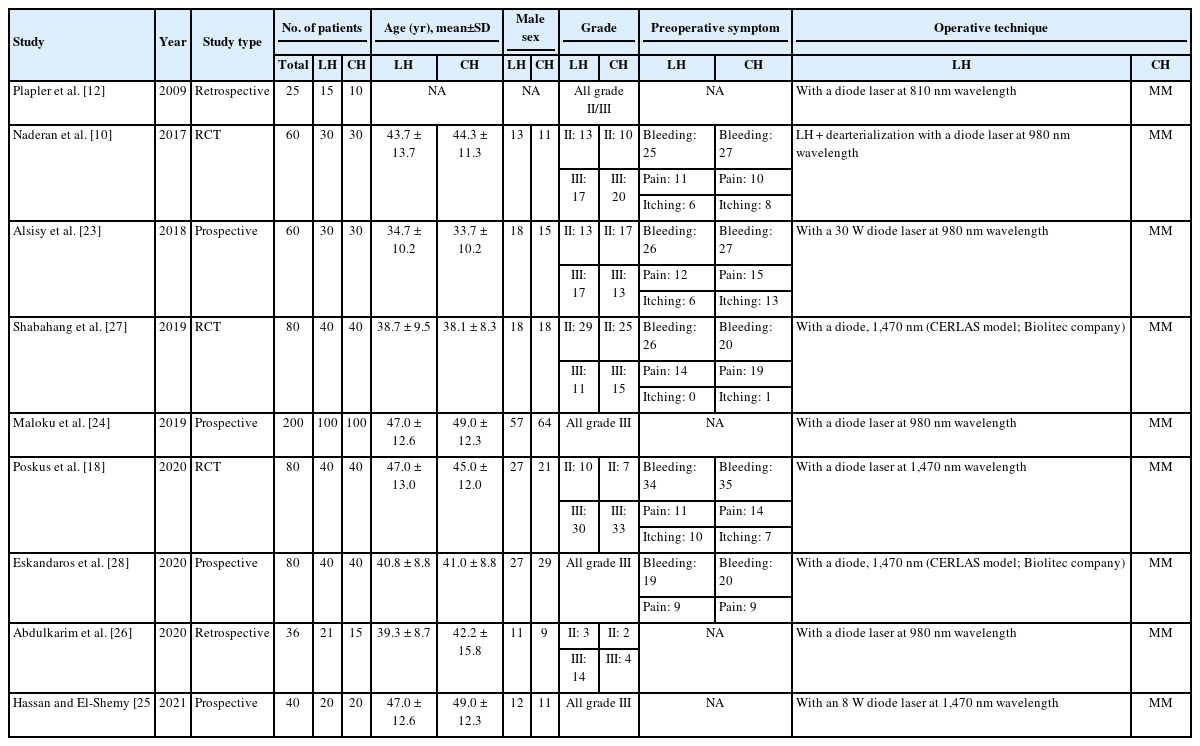
In recent years, laser hemorrhoidoplasty (LH) has emerged as a novel treatment modality. First described separately by Salfi [11] and Plapler et al. [12] in 2009, the early postoperative benefits have been demonstrated in comparison with other surgical methods, a likely result of the minimally invasive nature of laser therapy [12-17]. Thus far, 2 randomized controlled trials have made head-to-head comparisons between LH and conventional hemorrhoidectomy (CH), with encouraging outcomes. Patients in the LH arm had less postoperative pain and returned to regular activities earlier than those in the CH arm. At a 1-year follow-up, both trials reported comparable rates of symptom recurrence [10, 18].
Given emerging evidence from various trials and cohort studies, an analytical synthesis is timely. The aim of this paper was thus to perform a pairwise meta-analysis of real-world evidence comparing laser LH vs. CH for grade II or III hemorrhoids.
METHODS
Search process
This study was conducted in strict accordance with the Cochrane Handbook of Systematic Reviews and Meta-analysis ver. 6.2 (2021) [19] and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines [20]. In consultation with a research librarian, an electronic search was performed on August 22, 2021 in the following databases: MEDLINE (via PubMed), EMBASE, the Cochrane Library, and the ClinicalTrials.gov website to identify all published and indexed studies, and the gray literature comparing laser treatment vs. hemorrhoidectomy for grade II/III hemorrhoids. A repetitive and exhaustive permutation of the following Medical Subject Headings (MeSH) (expanded) terms were used: “laser,” “coagulation,” and “hemorrhoids.” The reference lists of relevant studies were manually searched to identify additional studies.
Inclusion and exclusion criteria
Both randomized controlled trials (RCTs) and non-RCTs were included if comparative outcomes were reported for patients undergoing upfront laser therapy or CH for grade II or III hemorrhoids. Single-arm, noncomparative studies were excluded. Non-English studies and English studies with no extractable data were excluded. Studies including grade IV hemorrhoids were excluded, as excisional hemorrhoidectomy currently remains the mainstay treatment method [4].
Selection of studies and data extraction
Studies were selected in 2 stages. First, 2 reviewers (IJYW, CHK) independently screened and assessed the studies for inclusion by their titles and abstracts. Full-text articles were subsequently obtained for review. The senior author served as the arbiter to resolve differences of opinion regarding the studies’ eligibility by consensus. The search strategy is shown in the PRISMA diagram (Fig. 1).
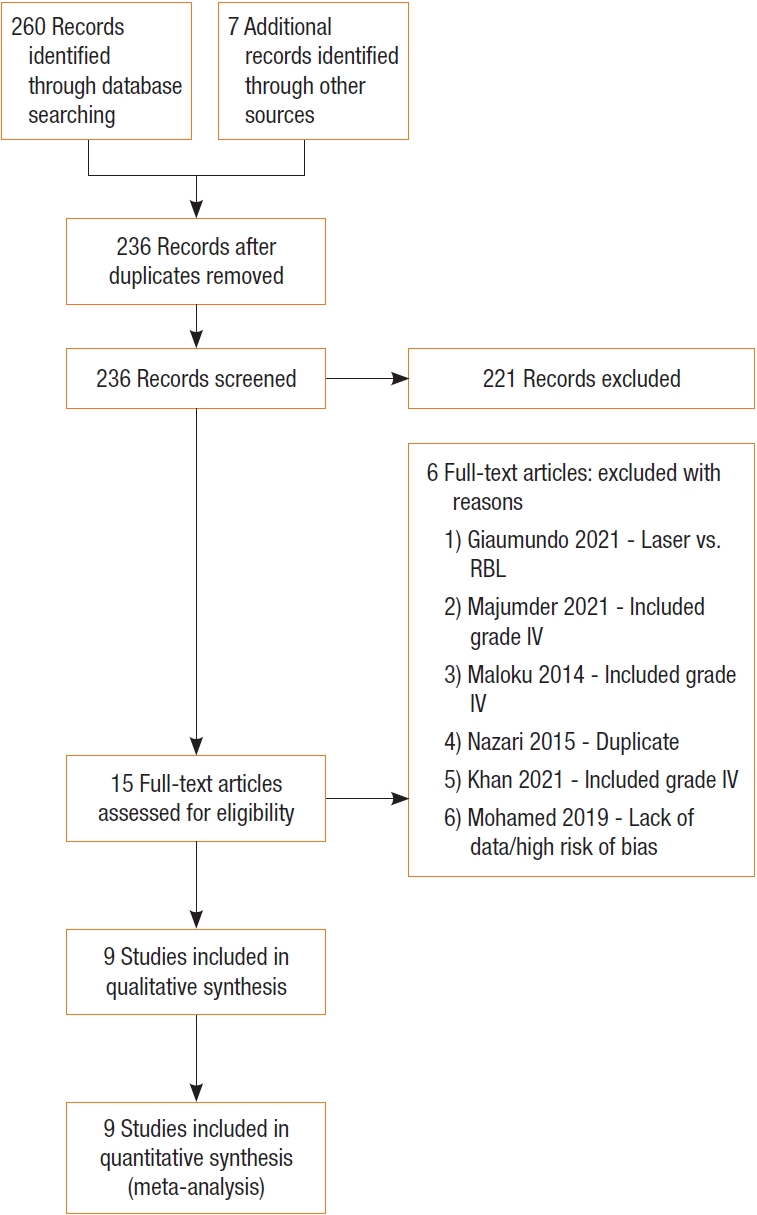
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart depicting systematic search process. RBL, rubber band ligation.
The outcomes of interest were divided into intraoperative and postoperative variables and extracted independently by 2 authors using a standardized proforma. The former included operative time and intraoperative blood loss. Postoperative variables included postoperative analgesia use, postoperative morbidity including bleeding, urinary retention, thrombosis, pain (measured via the visual analogue scale [VAS]), as well as the time of return to work/daily activities and longer-term symptom recurrence. Wherever reported, quality of life (QoL) scores were also assessed. In addition to the outcomes above, we extracted the following data from each study: first author, year, type of publication, age, sex, grade of hemorrhoids, and preoperative symptoms.
Statistical analysis
Meta-analyses were undertaken as per recommendations from the Cochrane Handbook [19]. Statistical analyses were performed using the RevMan 5.4 software (The Nordic Cochrane Centre), where pooling of weighted mean differences or standardized mean differences was conducted to generate summary statistics for continuous variables, while the risk ratio (RR) was used for dichotomous variables. Statistical heterogeneity was assessed using the I2 statistic. A random-effects model was chosen when the I2 statistic was greater than 50%, and a fixed-effects model otherwise. Results were reported with 95% confidence intervals (CIs), and a P-value of less than 0.05 was treated as statistically significant. Whenever variables were reported as median (range), the methods described by Hozo et al. [21] were implemented to convert them to the respective mean and standard deviation. To reduce heterogeneity, although subgroup and meta-regression analyses were considered, they were not performed given the small number of studies [19].
Assessment of bias
The Cochrane Risk of Bias tool was used to assess the study quality and risk of bias for RCTs based on aspects of selection, performance, detection, attrition, reporting, and other bias. For the quality of non-RCTs, the Newcastle-Ottawa Scale (NOS) [22] (for cohort studies) was utilized to assess the domains of patient selection, comparability of study groups, and outcome assessment. Publication bias was not assessed using the funnel plot and Egger regression test since there were fewer than 10 studies [19].
RESULTS
Systematic search
The systematic search across the various databases yielded an initial total of 260 publications. After removing duplicate publications and excluding those that did not fit the inclusion criteria based on title and abstract review, 15 publications remained and were reviewed in their entirety. Six publications were excluded for reasons stated in Fig. 1, while 9 [10, 12, 18, 23-28] were included after the final review, of which 3 were RCTs [10, 18, 27], 4 were prospective cohort studies [23-25, 28], and 2 were retrospective studies [12, 26]. Notably, of the 6 studies excluded, the decision to exclude that of Mohammed et al. [29] was largely based on a lack of clear data reporting and a high risk of reporting bias, despite a large sample size of 1,000 patients, which would have strengthened our study’s power and external validity.
Study characteristics
The 9 studies included 661 patients with grade II or III hemorrhoids, of whom 336 underwent LH and 325 underwent CH. The mean age of the LH and CH cohorts ranged from 34.7 to 47.0 years and 33.7 to 49.0 years, respectively. Males predominated in both arms. For studies that reported preoperative symptoms, the most common symptom was rectal bleeding, followed by pain and itching. Regarding the surgical technique, all studies employed conventional Milligan-Morgan (MM) hemorrhoidectomy. Detailed data can be found in Table 1.
Study quality
Of the 6 non-RCTs, all had a score of above 7 out of the maximum 9 on the NOS and were assessed to be robust methodologically, and of low risk of bias in terms of patient selection, comparability of study groups, and outcome assessment. Despite implementing surgical interventions, 3 RCTs had a low risk for performance and detection bias given the presence of blinding of participants, personnel, and outcome assessors (Supplementary Figs. 1, 2).
Intraoperative outcomes
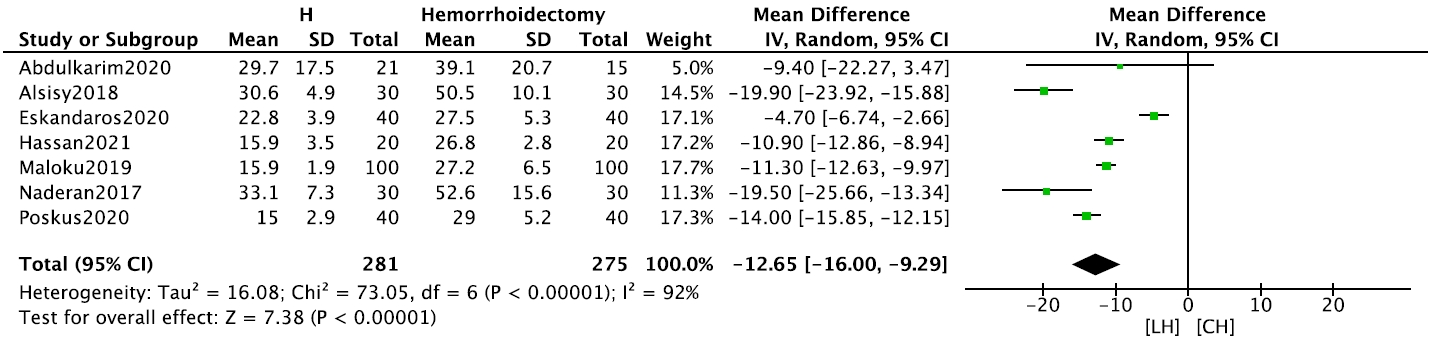
The LH group had a significantly shorter operative time (7 studies, n= 556) than the CH group (mean difference [MD], –12.65 minutes; 95% CI, –16.00 to –9.29 minutes; P< 0.001) (Fig. 2). In addition, blood loss (2 studies, n= 120) was significantly lower in the LH group than in the CH group (MD, –19.78 mL; 95% CI, –23.15 to –16.42 mL; P < 0.001) (Supplementary Fig. 3). Both meta-analyses were performed using fixed-effects models.
Early postoperative outcomes
In terms of postoperative morbidity, the risks of bleeding (6 studies, n= 520; RR, 0.18; 95% CI, 0.12–0.28; P< 0.001) (Fig. 3), and urinary incontinence (5 studies, n= 316; RR, 0.30; 95% CI, 0.10–0.95; P= 0.040) were significantly lower in the LH group (Supplementary Fig. 4). The pooled incidence of postoperative thrombosis was 0.07% across 3 studies that reported this outcome. Patients in the LH group returned to work or daily activities (4 studies, n= 420) significantly earlier than patients in the CH group (MD, –11.81 days; 95% CI, –19.39 to –4.23 days; P = 0.002) (Supplementary Fig. 5).

Forest plot comparing early postoperative bleeding between laser hemorrhoidoplasty (LH) and conventional hemorrhoidectomy (CH). CI, confidence interval; df, degree of freedom.
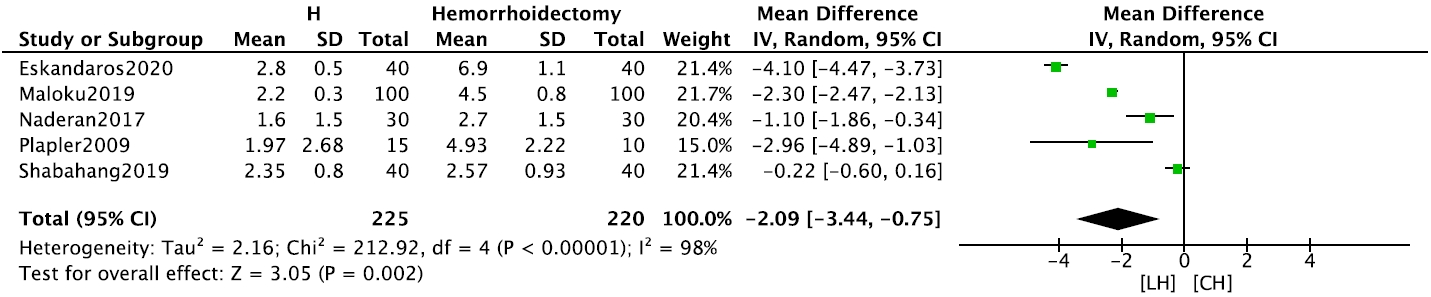
Concerning postoperative pain, the use of analgesia (3 studies, n= 160) was significantly lower in the LH group than in the CH group (RR, 0.59; 95% CI, 0.42–0.81; P= 0.001) (Supplementary Fig. 6). Only VAS scores reported on postoperative day (POD) 1 were meta-analyzable. Patients in the LH group had significantly lower POD 1 VAS scores (5 studies, n= 445) than those in the CH group (MD, –2.09; 95% CI, –3.44 to –0.75; P = 0.002) (Fig. 4). This remained consistent in the VAS scores 1 week postoperatively (2 studies, n = 105; MD, –3.94; 95% CI, –6.36 to –1.52; P= 0.001) (Supplementary Fig. 7).
Forest plot comparing early postoperative use of analgesia between laser hemorrhoidoplasty (LH) and conventional hemorrhoidectomy (CH). CI, confidence interval; df, degree of freedom.
Four studies, including those of Shabahang et al. [27], Eskandaros and Darwish [28], Maloku et al. [24], and Hassan and El-Shemy [25] reported longer-term VAS scores. However, these were not meta-analyzable due to heterogeneity in reporting. Shabahang et al. [27] reported no significant difference in VAS scores at 6 months postoperatively, despite a reduction in pain intensity in both groups (1.20± 0.40 in LH group vs. 1.37± 0.58 in CH group; P= 0.174). Eskandaros and Darwish [28] demonstrated a significantly lower VAS score in the LH group than in the CH group at 4 weeks (0.0± 0.0 vs. 1.2± 0.7, respectively; P< 0.001) and 8 weeks postoperatively (0.0 ± 0.0 vs. 0.4 ± 0.5, respectively; P < 0.001). Maloku et al. [24] showed that VAS scores were significantly lower in the LH group than in the CH group at POD 30 (0.2± 0.1 vs. 0.8± 0.2, respectively; P< 0.001) and POD 60 (similar values as POD 30). Hassan and El-Shemy [25] also reported significantly lower VAS scores in the LH group than in the CH group up to POD 30. Abdulkarim et al. [26] assessed pain symptoms postoperatively between the LH and CH groups and classified the pain scores as mild (85.7% vs. 66.7%, respectively), moderate (4.8% vs. 20.0%, respectively), and severe (9.5% vs. 13.3%, respectively), but found no significant differences (mean pain score of 1.05 vs. 2.00, respectively; P= 0.277).
Longer-term symptom recurrence and quality of life
Five studies [10, 18, 23-25] reported longer-term outcomes. Alsisy et al. [23] demonstrated that there were no statistically significant differences in terms of bleeding, pain, itching, and recurrence at 3 months of follow-up. Similar observations were noted by Maloku et al. [24] at 2 months of follow-up, where the incidence of bleeding was similar between both groups. Hassan and El-Shemy [25] also reported similar pain scores between both groups at 6 months of follow-up.
Patient follow-ups at 12 months were conducted in both RCTs included in this meta-analysis [10, 18]. There were no significant differences in the proportion of patients who remained completely asymptomatic (RR, 0.89; 95% CI, 0.74–1.08; P = 0.240) (Supplementary Fig. 8). Risks of bleeding (RR, 1.00; 95% CI, 0.42–2.37; P< 0.999) (Supplementary Fig. 9), and prolapse (RR, 1.71; 95% CI, 0.70–4.20; P= 0.240) were comparable (Supplementary Fig. 10).
Poskus et al. [18] and Shabahang et al. [27] were the only 2 researchers that formally conducted a QoL assessment using the 36 Item Short Form (SF-36) questionnaire. The former showed that the 1-year general health evaluation score was higher in the LH group than in the CH group (mean [interquartile range]: 60 [43–60] vs. 58 [50–70], P= 0.023). Furthermore, when patients were asked to evaluate the operation using a VAS from 1 to 10 at the 1-year visit, LH was regarded as the best operation by patients [18]. Corroborative findings were seen in the trial by Shabahang et al. [27], where the LH group reported better overall QoL than the CH group (P= 0.037).
DISCUSSION
This study is the first systematic review and meta-analysis specifically comparing LH against CH for grade II or III hemorrhoids. LH was demonstrated to have several advantages over CH both intraoperatively and postoperatively in the short as well as medium term. While a systematic review on laser treatment for hemorrhoidectomy was published by Lakmal et al. [30] in 2021, a meta-analysis was not performed given the inclusion of single-arm non-comparative studies.
From a technical perspective, LH can be performed more quickly and with less blood loss than CH. Patients who underwent LH experienced less postoperative pain and morbidity, enabling an earlier return to work or daily activities. Reduced pain is associated with improved patient satisfaction, fewer drug complications arising from analgesia use, and accelerated recovery [31]. In the medium term, at 1 year, postoperative morbidity and recurrence were not significantly different between both methods. One specific concern related to intrahemorrhoidal laser treatment is thrombosis of external hemorrhoids. However, the incidence of thrombosis was found to be low, and even if present, thrombosis can be managed successfully with medical treatment [10, 23, 26].
Despite the well-known short-term effects of CH, including significant postoperative pain, this technique does result in a low risk of symptom recurrence, at 2% to 8% at 1 year [32, 33]. Intuitively, an excisional procedure such as hemorrhoidectomy would have a lower recurrence rate compared to an ablative procedure, including LH, which does not involve tissue removal. While there was a trend toward higher hemorrhoidal symptom recurrence for LH compared to CH (28.6% vs. 20.0%) at postoperative 1 year, this result was not statistically significant. This finding may be explained by several underlying mechanisms. Laser therapy works by inducing hemorrhoidal tissue shrinkage and degeneration at varying depths [10]. At a molecular level, the laser causes submucosal protein denaturation and subsequent cellular fibrosis, followed by adherence to its underlying tissue, thereby preventing recurrent prolapse in the long term [17]. This achieves a similar effect of loss of hemorrhoidal tissue volume in CH, without physical tissue removal. Nonetheless, it should be emphasized that this result is the summation of only 2 RCTs. Further prospective trials with larger numbers of patients and a longer follow-up duration are required to draw definitive conclusions regarding hemorrhoidal recurrence rates between these modalities.
The findings from this study should be interpreted in the context of known limitations. The surgical method for CH was found to be homogeneous, as MM (open) hemorrhoidectomy was used universally in all studies. However, various outcomes showed both statistical and qualitative heterogeneity (e.g., for surgeon competency), which could not be eliminated completely using statistical tools. The inclusion of non-RCTs increased bias, which was addressed by risk assessment using the NOS score. Consequently, there are limitations arising from heterogeneity given the mixture of studies of various designs. In addition, the robustness of our results is limited by the small sample size. Given the small number of studies available, however, it was prudent to capture all available real-world evidence. The lack of homogeneous reporting of long-term data, in particular concerning hemorrhoidal symptom recurrence and patient QoL, precluded a holistic assessment of LH. Finally, further studies should be performed to compare laser therapy to other modalities of hemorrhoid surgery, which are known to be associated with lower postoperative pain than CH, including the Ferguson (closed) [34, 35], and Longo (stapled) hemorrhoidectomy [33]. Lastly, our results should be verified against future well-conducted RCTs with larger samples.
Despite being a nonexcisional procedure, LH results in improved clinical outcomes in the short term, with reduced morbidity, pain, and earlier return to work or daily activities, as well as similar medium-term symptom recurrence rates, when compared to CH. Follow-up data should be used to analyze longer-term symptom recurrence rates beyond 12 months. In addition, further trials should be performed comparing laser ablative therapy with other surgical approaches for hemorrhoids.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, Visualization: all authors. Investigation, Methodology, Data curation: IJYW, CHK, ISE. Formal analysis: IJYW. Project administration, Supervision: EJKWT, ISE. Writing–original draft: IJYW, CHK, ISE. Writing–review & editing: all authors.
All authors have read and approved the final manuscript.
SUPPLEMENTARY MATERIALS
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00598.0085.
Supplementary Fig. 1.Risk of bias summary of 3 randomized controlled trials.
Supplementary Fig. 2.Risk of bias graph of 3 randomized controlled trials.
Supplementary Fig. 3.Intraoperative blood loss.
Supplementary Fig. 4.Risk of postoperative urinary retention.
Supplementary Fig. 5.Time to return of daily activity.
Supplementary Fig. 6.Use of analgesia.
Supplementary Fig. 7.Visual analogue scale scores of postoperative day 7.
Supplementary Fig. 8.Twelve-month symptom free.
Supplementary Fig. 9.Twelve-month risk of bleeding.
Supplementary Fig. 10.Twelve-month risk of prolapse.