- Search
|
|
Abstract
Purpose
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been proposed for controlling peritoneal seeding metastasis in some kinds of cancers, including those of colorectal origin, but their safety and oncological benefits are subjects of debate. We present our early experience with those procedures.
Methods
Data were retrospectively collected from all patients with peritoneal carcinomatosis (PC) and pseudomyxoma peritonei (PMP) treated using CRS and HIPEC at Yonsei Cancer Center between July 2014 and July 2015. Short-term outcomes and risk factors for postoperative complications were analyzed.
Results
Twenty-three patients with PC (n = 18) and PMP (n = 5) underwent CRS and HIPEC. Median follow-up and age were 2 months and 54 years, respectively. The median peritoneal carcinomatosis index score was 15, and CC0-1 was achieved in 78.3% of all patients. The median operation time and bleeding loss were 590 minutes and 570 mL, respectively. Grade-IIIa/grade-IIIb complications occurred in 4.3% (n = 1)/26.1% (n = 6) of the patients within 30 days postoperatively, and no 30-day mortalities were reported. Factors related to postoperative complications with CRS and HIPEC were number of organ resection (P = 0.013), longer operation time (P < 0.001), and amount of blood loss (P = 0.003). All patients treated with cetuximab for recurred colorectal cancer had grade-III postoperative complication.
Peritoneal carcinomatosis (PC) is not an uncommon condition encountered in patients with colorectal cancer and, based on epidemiologic studies, is known to occur in approximately 10%ŌĆō15% of all patients [1]. The median survival expectancy for patients with PC was reported to be about 7ŌĆō8 months in untreated patients [2] and 12 months even in patients treated with systemic chemotherapy [3] until several centers worldwide reported favorable survival outcomes for patients treated using combined cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
Even though the use of a combination of CRS and HIPEC involves considerable risk of complications, as has been reported in several cohort studies and case series (29%ŌĆō56%) [4,5,6,7], its efficacy in selective patient groups is hardly in dispute. Verwaal [3] reported fascinating survival outcomes in their randomized controlled trial: a median survival of 22.3 months and a significant risk reduction of dying (hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.32ŌĆō0.95; log-rank P = 0.032) in the HIPEC group in comparison to the systemic chemotherapy only group. Moreover, this substantial overall survival is likely to be amplified further when patients to be treated using combined CRS and HIPEC are very carefully selected. Elias et al. [8], one of the leading groups using CRS and HIPEC, reported that patients with isolated, resectable PC could prolong their median survival by up to 63 months with this aggressive procedure in comparison to a median survival of 24 months with modern chemotherapies. Sugarbaker [9] insisted that a subset of patients with a peritoneal carcinomatosis index (PCI, which quantifies the intraperitoneal tumor burden by distribution and lesion size with a range of 0ŌĆō39) less than 20 could profit in survival from his experience, and he emphasized the completeness of cytoreduction (CC 0ŌĆō1) for the best survival outcome.
Recently, some leading experts even asserted the necessity for using prophylactic HIPEC. They claimed that early peritoneal metastases are impossible to detect given the absence of symptoms and current limitations of imaging; therefore, proper timing of surgical intervention could be delayed. Indeed, some European centers are performing clinical randomized trials to demonstrate the role of prophylactic HIPEC in selective high-risk subgroups of patients with peritoneal recurrence [10].
In Korea, some centers have been performing combined CRS and HIPEC as an alternative treatment for patients with synchronous or metachronous colorectal cancer with PC since HIPEC was first authorized as a new medical technology by the Ministry of Health and Welfare in late 2013. Even though HIPEC is being widely used for the treatment of PC in Western Europe and the United States, it is still known to involve high morbidity even at centers with experienced surgeons. Furthermore, HIPEC-related short-term outcomes in Korea are not available yet. Therefore, in this paper, we present and discuss our early experience related to HIPEC.
Data were retrospectively collected from all patients with PC and pseudomyxoma peritonei (PMP) treated by using of combination of CRS and HIPEC at Yonsei Cancer Center between July 2014 and July 2015. HIPEC was first introduced and conducted at our institution in July 2014. CRS was performed with intention-to-treat in all patients for attaining R0-1 resections. Data were analyzed to demonstrate the safety of this procedure and the factors related with perioperative adverse events.
All the patients referred to our clinic for CRS and HIPEC were thoroughly evaluated in order to exclude possible systemic metastasis other than peritoneal seeding and to determine the tumor burden of PC and PMP. Diagnostic work-ups included esophagogastroduodenoscopy and colonoscopy, as well as computed tomography (CT) scans of the chest, abdomen and pelvis with IV contrast agents. Positron-emission tomography-CT (PET-CT) was considered if extra-abdominal metastasis was suspected or was difficult to determined based on the CT scans.
Patients with synchronous PC originating from colorectal cancer without systemic metastasis were mostly managed by using a combination of CRS and HIPEC, but patients with metachronous PC, which was incidentally detected by serial elevation of serum carcinoembryonic antigen (CEA) or on a CT scan during the follow-up period after a curative resection, were initially considered as candidates for systemic chemotherapy. The cycles of preoperative chemotherapy were left to the oncologist's decision, but the resectability was discussed by the surgeon and the radiologist every 4ŌĆō6 cycles of chemotherapy. Also, if the patient requested other treatment options rather than chemotherapy or the oncologist judged continuation of chemotherapy to be impossible because of its toxicity and low patient compliance with the treatment, a multidisciplinary team approach was recommended to the patient, the treatment plan was discussed further, and finally a decision was made to perform CRS and HIPEC when the tumor was such that a complete resection was thought to be possible, provided that the patient would benefit from the procedure. At out institution, PMP with obstructive symptoms or impending obstruction was considered to be an indication for the use of combined CRS and HIPEC.
Preoperative diagnostic laparoscopy was not routinely performed for the evaluation of resectability. Its use was entirely left to the surgeon. Mostly, the way to proceed for the CRS and HIPEC was decided under direct vision after a long midline abdominal incision had been made from the xiphoid process to the symphysis pubis. The extent of PC was determined at the time of initial surgical exploration by using the PCI score. The PCI score, which was first suggested for the staging of PC by Jacquet and Sugarbaker [11] in 1996 and which comprises 13 abdominopelvic regions with lesion size scores and is a summed numerical score from 0 to 39, was adopted at our institution to investigate the extent of PC and to help surgeons determine how to apply the procedure. However, the indications for the use of CRS and HIPEC at our institution do not coincide with Sugarbaker [9]'s decision algorithm, but rather are based on those agreed on at a 2006 HIPEC consensus meeting in Milano, Italy [12]. In other words, a combination of CRS and HIPEC is used when complete cytoreduction is possible, regardless of the PCI score.
CRS included removing the primary tumor with acceptable resection margins, metastatic lymph nodes, involved organs, and all peritoneal seeding metastases in the abdominopelvic cavity. If the tumors were unresectable because of anatomical location, including mesenteries of the small and the large bowels, a high-voltage monopolar device was used for cauterization of multiple small lesions. A peritonectomy was not conducted in any of the patients; rather, a peritonectomy was performed if visual peritoneal seeding metastases were confirmed at the initial surgical exploration. Before HIPEC was started, the completeness of cytoreduction (CC score from 0 to 3) [9] was measured and recorded.
HIPEC was facilitated using the open (coliseum) technique, which was prepared by suturing 1-0 vicryl to the deep layer of the epidermis and subcutaneous area and fixing it to an Omni retractor with mosquito instruments. Initially, 3 L of heated perfusion solution was infused into the abdominal cavity at a rate of 600ŌĆō800 mL/min through the inflow tube by way of a Belmont hyperthermic pump. When the temperature of the abdominal cavity had reached 40ŌäāŌĆō41Ōäā, the first dose of mitomycin-C of 17.5 mg/m2 was mixed into the heated solution, after which a dose of 8.8 mg/m2 was added every 30 minutes. The temperature of the perfusion solution was maintained and evenly distributed at 41ŌäāŌĆō42Ōäā by stirring with a surgeon's hand. The duration of the HIPEC procedure was 90 minutes, after which the perfusion solution was completely drained and a bowel anastomosis was performed, if needed. All patients were taken to the intensive care unit for short-term observation.
Adjuvant chemotherapeutic regimens were mainly with FOLFOX (oxaliplatin, leucovorin and 5-FU) or FOLFIRI (irinotecan, leucovorin and 5-FU) with or without biologic agents. Postoperative chemotherapy was recommended for all patients after they had completely recovered from surgical stress without any complication.
After the completion of postoperative chemotherapy, clinical evaluations were performed regularly every 3 months for the first 2 years and thereafter every 6 months for the subsequent 3 years. Serum CEA was measured whenever the patient visited the outpatient clinic, and CT scans of the chest, abdomen and pelvis were performed every 6 months during the follow-up period.
Patients were grouped according to the Clavien-Dindo classification [13]. Group A, which was grades 0ŌĆōII, included patients with mild complications without any need for further intervention, and group B, which was more than grade III, included patients with moderate-to-severe complication with need for surgical exploration or radiological/medical intervention. Each factor associated with a postoperative complication after CRS and HIPEC was divided into 2 groups by using the cutoff value from the receiver operating characteristic curve and was analyzed using the chi-square test. All P-values were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were carried out using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
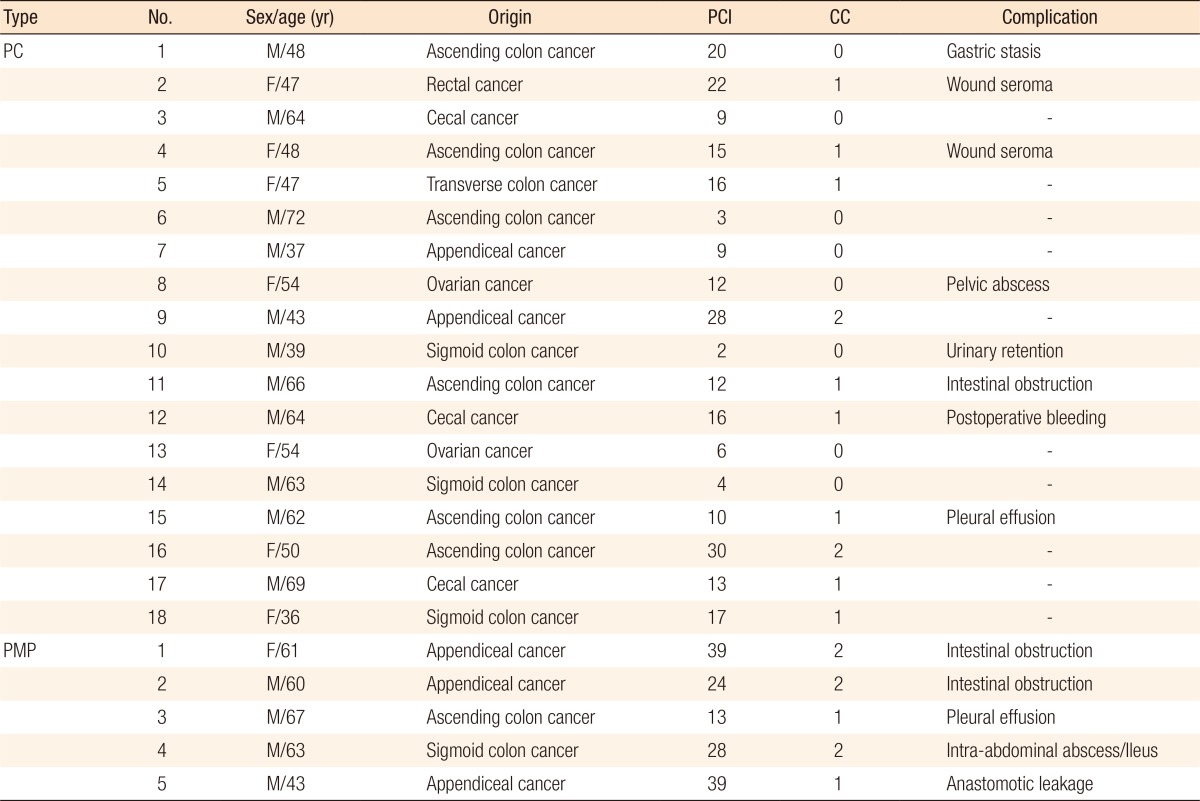
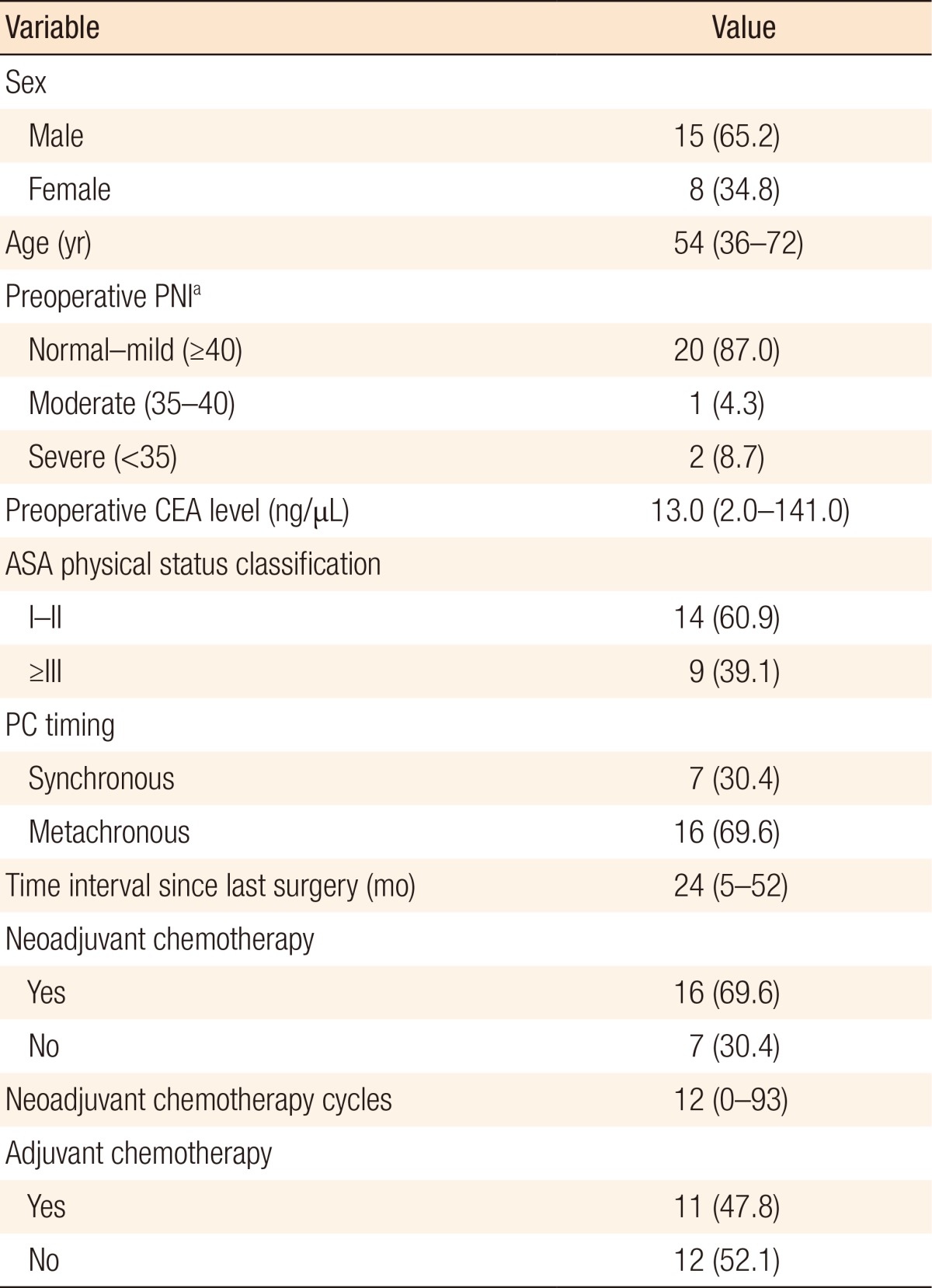
A total of 23 patients with PC (n = 18) and PMP (n = 5) were considered for CRS and HIPEC with intention-to-treat (Table 1). The median follow-up period was 2 months (range, 0ŌĆō11 months), and the median age was 54 years old (range, 36ŌĆō72 years). Among these patients, 39.1% (n = 9) had an American Society of Anaesthesiologists physical status classification grade of more than III, and 13% (n = 3) were moderate-to-severe malnutrition status. All the patients with synchronous PC had a CRS and HIPEC without any neoadjuvant chemotherapy. However, patients with metachronous PC mostly had undergone preoperative chemotherapy, a median of 12 cycles (range, 0ŌĆō93 cycles) for 24 months (range, 5ŌĆō52 months), during the time from their last surgery until the CRS and HIPEC was performed (Table 2).
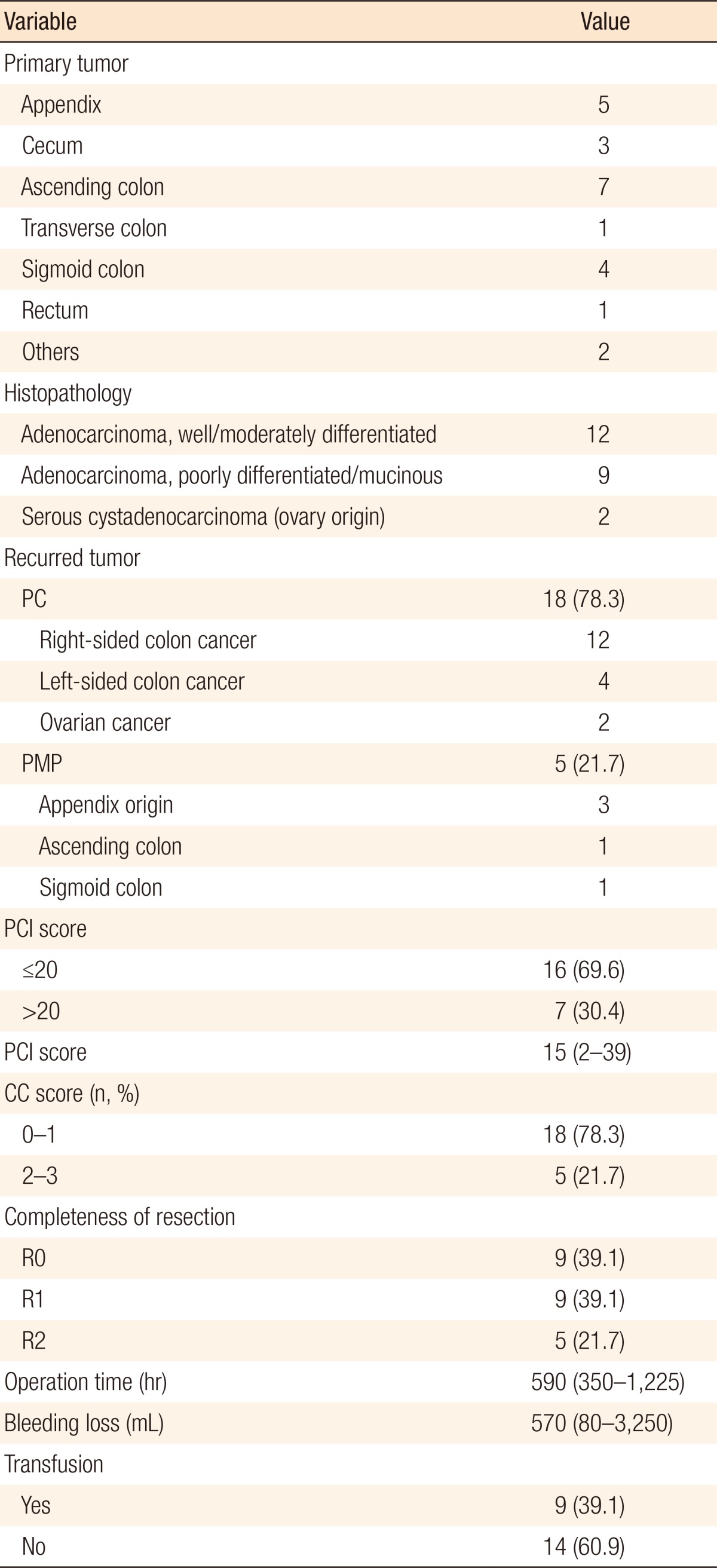
The primary tumor origin of PC in patients with colorectal cancer was mostly the right-side colon (75%, 12 out of 16). The median PCI score was 15 (range, 2ŌĆō39), and patients with PCI scores of more than 20 were included in this study (n = 7, 30.4%). Complete cytoreduction was possible in 78.3% (n = 18) of the patients, but in 21.7% (n = 5) of the patients, the resection was an R2 resection. The median operation time and the bleeding loss were 590 minutes (range, 350ŌĆō1,225 minutes) and 570 mL (range, 80ŌĆō3,250 mL), respectively, and 9 patients (39.1%) required a transfusion during operation (Table 3).
Most patients recovered and were discharged a median of 19 days (range, 8ŌĆō101 days) after surgery. Adverse events during the first 30 days following surgery occurred in 12 (12 of 23, 52.2%) patients, and among those 12, 7 (7 of 12, 58.3%) needed further radiological intervention or surgical exploration. Moreover, late complications occurred in 2 of the 23 patients (8.7%), for whom delayed surgical intervention was needed. Therefore, overall 9 of the 23 patients (39.1%) who underwent CRS and HIPEC had more than grade-III complications according to the Clavien-Dindo classification [13]. No mortalities occurred during the first 30 days of the follow-up period, but disease-related deaths occurred in 3 patients (13.0%) (Table 4).
Factors such as organs resected (more than 3), longer operation time (Ōēź 630 minutes), and large amount of blood loss (Ōēź600 mL) during surgery were found to be associated with a high rate of postoperative complications. Interestingly, all 4 patients with recurred colorectal cancer who had been treated with cetuximab had grade-III postoperative complications. However, anastomotic leakage occurred in only 1 patient, who had a complete total colectomy with ileorectal anastomosis and HIPEC because of PMP. Preoperative nutritional status and number of bowel anastomoses were not closely related to the occurrence of postoperative adverse events (Table 5).
Before HIPEC was permitted for the treatment of patients with colorectal cancer with PC in Korea, the only option for patients with PC was systemic chemotherapy with or without biologic agents. Even though newly developed chemotherapeutic and biologic agents have prolonged the life expectancy in those patients [14], chemotherapy-related adverse events or intolerance and resistance to this therapy ultimately made it impossible to prevent tumor progression and led to withdrawal of the treatment.
Our institution initially had difficulty in enrolling patients for CRS and HIPEC because our oncologists and gastroenterologists had some suspicions about this new procedure with regards to its efficacy and safety. This distrust made them hesitate to introduce this new therapeutic option to their patients in whom the disease had been stabilized with continuous chemotherapy. The high complication rates previously reported in many centers worldwide [4,5,6,7] and the existence of only a few randomized controlled trials were the main obstacles to persuading them to enroll their patients who were suffering from metachronous PC. Therefore, the first enrolled patient was one who had colorectal cancer with synchronous PC and who had been directly referred to our department by a local clinic. This patient was initially diagnosed with ascending colon cancer with a few peritoneal seeding nodules based on the CT scan (PCI score, 8), but the intraoperative PCI score eventually revealed more than what had been expected preoperatively (intraoperative PCI score, 20). Even though this patient temporarily had gastric stasis, he recovered completely three weeks later and received adjuvant chemotherapy afterwards. He has shown no evidence of tumor recurrence since then. After the first patient had recovered without any major complication and HIPEC had been shown not to delay adjuvant chemotherapy, patients with metachronous PC started to be enrolled in HIPEC therapy by oncologists and gastroenterologists. As Tables 1 and 2 show, not only patients with PC (n = 2) originating from other sites such as the ovaries but also patients with PMP (n = 5) were included in the analysis because the main purpose of our study was to introduce our early experiences with respect to the safety of this new procedure in a single center.
As for HIPEC-associated complications, 3 adverse events (3 of 7, 14.3%), urinary retention, gastric stasis, and intestinal obstruction, occurred in patients with synchronous PC, and the patient with intestinal obstruction was eventually treated with surgical intervention. To be frank, this patient was the first and the last patient enrolled in HIPEC despite his having a nonprogressive single hepatic metastasis after completion of 12 cycles of neoadjuvant chemotherapy, which is known to be a contraindication for HIPEC. During a 660-minute surgery, CRS and intraoperative radio-frequency ablation to a single hepatic metastasis were performed, attaining a R0 resection. Among 16 patients with metachronous PC or PMP, 9 patients (56.3%) had postoperative complications within 30 days. Excluding 3 patients with minor adverse events, 6 other patients with major complications (37.5%), such as intra-abdominal abscess, pleural effusion, intestinal obstruction, immediate postoperative bleeding and anastomotic leakage, were finally treated with radiologic or surgical interventions.
Given our short-term outcomes, the major complication rate for patients with synchronous PC tended to be lower than it was for patients with metachronous PC. This is the reason patients with metachronous PC commonly present with conditions that are unfavorable for surgery, including severe adhesion, undernutritional status and previous multiple cycles of chemotherapy with or without biologic agents. As Table 5 shows, all 4 patients treated with cetuximab preoperatively had major complications, such as pleural effusion, intestinal obstruction, immediate postoperative bleeding and intra-abdominal abscess with sepsis. In a recent European study, bevacizumab was also found to be associated with a twofold increased morbidity in patients treated with CRS and HIPEC (odds ratio, 2.28; 95% CI, 1.05 to 4.95; P = 0.04) [15]. Therefore, great attention is needed when performing CRS and HIPEC, particularly in the patients who have metachronous PC or have received preoperative chemotherapy or biologic therapy.
In addition to the aforementioned preoperative unfavorable conditions, the major complications tended to be associated with several intraoperative factors, as shown in Table 5. Major complications occurred more commonly in patients who had more than 3 organs resected, operation times of 630 minutes or longer, and losses of 600 mL or more of blood during surgery. Against our expectation, anastomotic leakage occurred in only one patient (4.3%) and showed no significant differences compared to usual colorectal surgery. A great diversity of opinion exists concerning this early postoperative major complication rate (7 of 23, 30.4%), i.e., whether it needs to be abided for better oncologic outcomes or not. However, if we can attain the same oncologic outcomes as leading experts in Europe and the United States do, we think this aggressive procedure, with its accompanying high morbidity, should be considered for carefully selected patients who might benefit from it use.
In conclusion, our initial experience with CRS and HIPEC showed that about 30% of the patients experienced grade-III postoperative complications, which was strongly associated with the number of organs resected, longer operation time, and the amount of blood loss. Therefore, an expert surgeon needs to perform this combined procedure with great caution and only on selected patients who might benefit from it.
References
1. Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545ŌĆō1550. PMID: 12445064.


2. Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 2011;128:2717ŌĆō2725. PMID: 20715167.


3. Verwaal VJ. Long-term results of cytoreduction and HIPEC followed by systemic chemotherapy. Cancer J 2009;15:212ŌĆō215. PMID: 19556907.


4. van Leeuwen BL, Graf W, Pahlman L, Mahteme H. Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol 2008;15:745ŌĆō753. PMID: 18057988.


5. Cashin PH, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol 2012;38:509ŌĆō515. PMID: 22475555.


6. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737ŌĆō3743. PMID: 14551293.


7. Huang CQ, Feng JP, Yang XJ, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol 2014;109:730ŌĆō739. PMID: 24374987.


8. Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681ŌĆō685. PMID: 19103728.


9. Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999;43(Suppl): S15ŌĆōS25. PMID: 10357554.


10. Baratti D, Kusamura S, Deraco M. Prevention and early treatment of peritoneal metastases from colorectal cancer: second-look laparotomy or prophylactic HIPEC? J Surg Oncol 2014;109:225ŌĆō226. PMID: 24452990.


11. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359ŌĆō374. PMID: 8849962.


12. Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol 2007;14:128ŌĆō133. PMID: 17072675.


13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205ŌĆō213. PMID: 15273542.



14. Klaver YL, Leenders BJ, Creemers GJ, Rutten HJ, Verwaal VJ, Lemmens VE, et al. Addition of biological therapies to palliative chemotherapy prolongs survival in patients with peritoneal carcinomatosis of colorectal origin. Am J Clin Oncol 2013;36:157ŌĆō161. PMID: 22314003.


15. Eveno C, Passot G, Go├®r├® D, Soyer P, Gayat E, Glehen O, et al. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1792ŌĆō1800. PMID: 24337648.


Table┬Ā5
Factors associated with postoperative complications after CRS + HIPEC
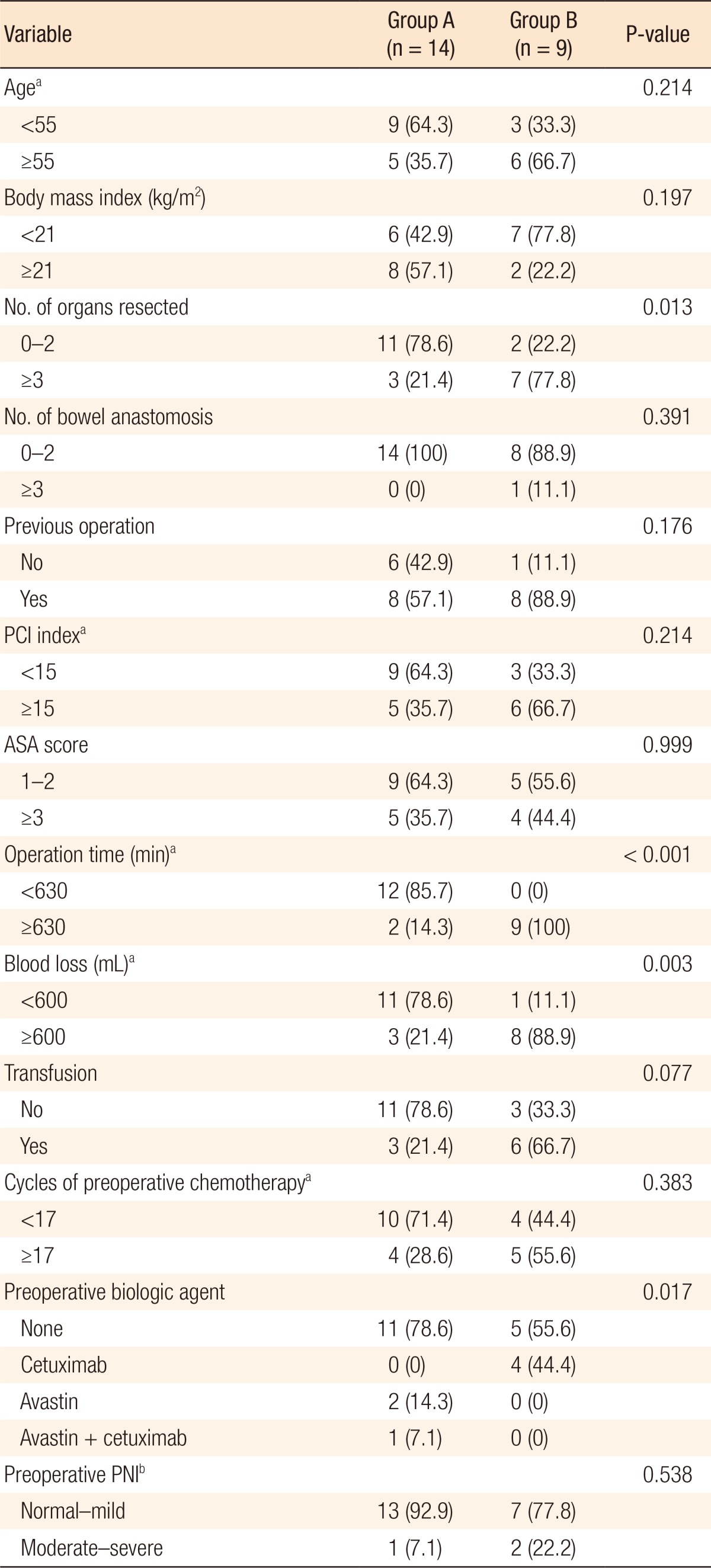
CRS + HIPEC, cytoreductive surgery + hyperthermic intraperitoneal chemotherapy. Group A, Clavien-Dindo classification grades 0ŌĆōII; group B, Clavien-Dindo classification grade Ōēź III.
aCutoff value obtained from the receiver operating characteristic curve. bPrognostic nutrional index (normal, Ōēź50; mild, 40ŌĆō50; moderate, 35ŌĆō40; severe, <35).
- TOOLS