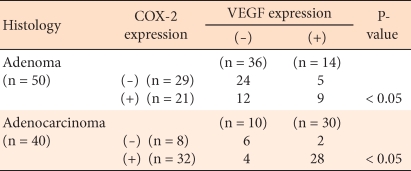- Search
Abstract
Purpose
Recent studies have shown that cyclooxygenase (COX)-2 may be involved in tumor growth, invasion and apoptosis in various carcinomas. Vascular endothelial growth factor (VEGF) has a potent angiogenic activity, and COX-2 promotes angiogenesis by modulating angiogenic factors, including VEGF. Endothelial growth factor receptor (EGFR) is considered as a factor of cell growth, maturation and cell death. The current study was designed to investigate the possible roles of COX-2 in colorectal tumor progression and angiogenesis.
Methods
Fifty colorectal adenomas and forty adenocarcinomas were investigated by using immunohistochemical staining for COX-2, VEGF and EGFR. The correlations of COX-2, VEGF and EGFR with the grade of dysplasia, the size of the adenoma, and various clinicopathologic factors were studied.
Results
The expressions of COX-2, VEGF and EGFR were each significantly correlated with carcinomatous transformation, and the expressions of COX-2 and VEGF were significantly correlated. COX-2 and EGFR showed correlations with adenomas rather than adenocarcinomas. However, no correlations of COX-2, VEGF and EGFR expression to other clinicopathologic factors, except tumor size in EGFR expression, were detected.
The concentration of prostaglandin (PG) has been found to be increased in several cancers, and studies on the effect of PG and its metabolites on the growth and metastasis of cancer are ongoing actively [1-3]. PG is produced through the action of cyclooxygenase (COX), the rate limiting enzyme of the arachidonic acid metabolism. COX is a key enzyme converting arachidonic acid to eicosanoids, including PG, and the produced PGs mediate complex biological functions in vivo [2, 4]. COX can be classified into two isozymes, COX-1 and COX-2. COX-1 is expressed in a constant concentration in most normal cells, and it is involved in the protection of the gastric mucosal epithelium, the maintenance of renal plasma and the regulation of platelet aggregation. COX-2 is rarely expressed in normal cells, it is induced by stimulations of cytokines, growth factors, hormones, mitosis, and it has been revealed to be expressed in inflammatory cells or cancer cells. COX-2 selective inhibitors have been used as therapeutic agents for arthritis without pre-existing gastrointestinal disturbance or anti-inflammatory agents [4, 5].
In previous experimental studies, nonsteroidal antiinflammatory drugs (NSAID) have been reported to reduce the incidence of colon polyps and colon cancers [6, 7], induce the degeneration of breast cancer [8], and suppress the growth of tumors in the neck [9]. The mechanism of NSAID suppressing the development of colon cancer has not been elucidated yet; nonetheless, it is thought to reduce the production of PG by interfering with COX activity and to exert effects on cell growth, apoptosis and immune reactions [3, 10]. Nevertheless, clinical studies have revealed that COX inhibitors, including NSAID, are restricted to use as therapeutics because of gastrointestinal disturbance and cardiovascular impairments [11].
Recently, correlations between the expression of COX-2 and other oncogenic factors associated with tumor growth and distant metastasis have become an issue; thus, attention has been paid to COX-2 as a prognostic factor. The overexpression of COX-2 has been reported to affect the regulation of the angiogenesis of tumors by being involved in the synthesis of various angiogenic factors, such as vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), and thus to play an important role in the growth and progression of tumors [12, 13]. Tumor angiogenesis is an important factor for tumor growth, infiltration and metastasis (when solid tumors grow, they induce vascular stroma), and the level of vascularized stroma has been reported to be closely associate with prognosis [14]. Among several angiogenesis factors, VEGF and EGFR have been reported to be overexpressed not only in colorectal cancer but also in several other tumors [15-17].
In stomach cancer, breast cancer, head and neck cancer, and colorectal cancer, several studies on the association of COX-2, VEGF, and EGFR with angiogenesis have already been reported [13, 15-18]. Nonetheless, such reports on colorectal adenomas and carcinomas are rare; thus, in our study, the expressions of COX-2, VEGF, and EGFR in colorectal adenomas and carcinomas were examined by using immunohistochemical staining to find the differences in the expressions according to the histological differentiation grade and the tumor size. Also, whether COX-2 is associated with angiogenesis and thus plays a role in the development and growth of colorectal cancer and the mechanism of metastasis, and its role as a prognostic factor were investigated.
Patients with pathologically proven colorectal adenomas or carcinomas from January 2003 to June 2004 at the National Health Insurance Corporation, Ilsan hospital, were included in this study. Fifty patients on whom a polypectomy or an endoscopic mucosal resection had been performed were selected from among the colorectal adenoma patients admitted to the Department of Gastrointestinal Medicine, and 40 patients were selected from among colorectal cancer patients who underwent surgery in the Department of Surgery. Among the 50 cases of colorectal adenoma, 38 cases were low grade dysplasia and 12 cases showed high grade dysplasia; 43 cases were tubular adenomas, 6 cases were tubulovillous adenomas, and 1 case was a serrated adenoma. Among the 38 cases showing low grade dysplasia, 15 patients had lesions smaller than 5 mm, and 23 patients had lesions larger than 5 mm.
The clinical records of the patients were examined, and clinical findings associated with prognosis, such as age, gender, clinical stage, etc., were examined. By reviewing pathological reports and slides with H&E staining, the sizes and locations of the lesions in the extracted tissues were assessed and histological types, differentiation grades, and the presence or absence of adjacent lymph node metastasis were reconfirmed. The pathological stage was classified according to the TNM stage of the American Joint Committee on Cancer.
The most appropriate paraffin block of each case was selected, and according to the avidin-biotin complex method, immunohistochemical staining for COX-2, VEGF, and EGFR was performed. Paraffin-embedded samples were sectioned, deparaffined, rehydrated with ethanol, and treated with 3% hydrogen peroxide solution for 10 minutes at room temperature to suppress endogenous peroxidase. Antigen retrieval was performed by using microwaves. The samples were treated with blocking antibody and were subsequently incubated with the primary antibody to COX-2, EGFR (1:100, Zymed, San Francisco, CA, USA) or VEGF (1:400, BD Pharmingen, San Diego, CA, USA) at 4Ōäā overnight. On the next day, the samples were washed with Tris buffer for 10 minutes, treated with the secondary antibody and streptavidin-peroxidase conjugate, stained with diaminobenzidine, counterstained with Mayer's hematoxylin, and examined under a microscope. The results were interpreted by two pathologists independently. If their interpretations were not in agreement, they re-examined the corresponding slide together and made a determination.
The staining of COX-2 was scored as follows: 0, cells without staining in the cytoplasm; 1, less than 50% of the cells with mild or moderate staining in the cytoplasm; 2, more than 50% of the cells with mild or moderate staining or less than 50% of the cells with strong staining in the cytoplasm; 3, more than 50% of the cells with strong staining in the neoplasm. Cases with more than 2 points were determined as positive. The interpretations of the results of VEGF or EGFR staining were based on a semi-quantitative analysis. The samples were divided into 4 levels: cases were 0 points, cases with less than 10% positive cells were 1 point, cases with 10-50% positive cells were 2 points, and cases with more than 50% positive cells were 3 points. Cases with more than 2 points were considered to be positive. The positive control for COX-2, VEGF, and EGFR was gastric adenocarcinoma, and the negative control was the normal mucosa around tumors on the same slide.
In colorectal adenomas and carcinomas, COX-2 was primarily expressed in the cytoplasm as a diffuse or microgranular pattern. The pattern was the weak staining of some adjacent interstitial cells or inflammatory cells and was not detected in the normal mucosal epithelium (Fig. 1A). Particularly, in cancer cell clusters, the infiltrative border showed stronger staining patterns than the center area (Fig. 1B).
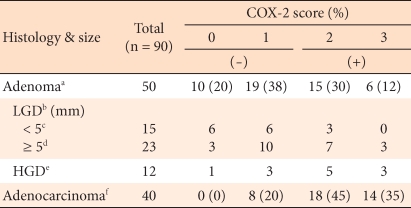
In regard to the expression levels of COX-2 in the 50 cases of colorectal adenomas, 10 cases had 0 points, 9 cases had 1 point, 15 cases had 2 points, and 6 cases had 3 points. According to the differentiation grade of the adenoma, the COX-2 positive rate in the high grade dysplasia cases was 66.7%, and it was shown to be higher than the 34.2% for low grade dysplasia cases. Among the low grade dysplasia cases, according to the size of the tumor, the COX-2 positive rate in tumors with sizes larger than 5 mm was 43.4%, and that in tumors with sizes smaller than 5 mm was 20%, but this difference did not show statistical significance (P > 0.05). The COX-2 positive rate in colorectal carcinomas was shown to be high, 80%, and it was statistically significantly higher than the 44% positive rate for adenoma cases (P < 0.05) (Table 1).
In colorectal adenomas and carcinomas, VEGF was expressed limitedly in the cytoplasm and cell membrane, and it was stained more strongly in the infiltrative area of the carcinoma (Fig. 2). In addition, it was stained weakly in vascular endothelial cells, inflammatory cells, and interstitial cells.
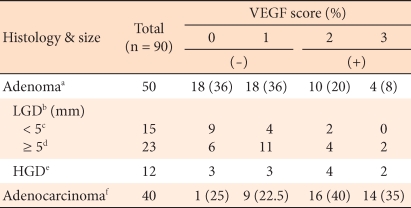
The positive expression rate of VEGF in colorectal adenomas was 28%, and in colorectal carcinoma, it was 75%, this difference being statistically significant (P < 0.05). Similar to COX-2, the expressions according to the histological dysplasia level of colorectal cancer or according to tumor size did not show significant differences (P > 0.05) (Table 2). In colorectal adenomas, the positive rate in high grade dysplasia cases was 50%, and in low grade dysplasia cases, it was 26.4%. In low grade dysplasia cases, according to tumor size, the positive rate in cases with sizes larger than 5 mm was 26.1%, and that in cases with sizes smaller than 5 mm was 13.3%.
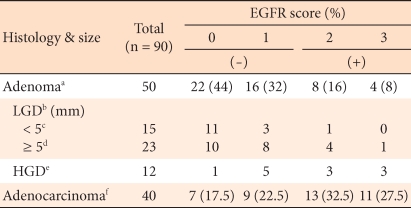
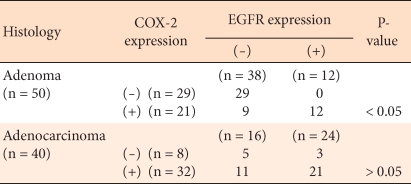
In colorectal adenomas and carcinomas, EGFR was expressed limitedly in the cytoplasm and cell membrane of tumor cells. In adenomas, it was expressed weakly, or only in some areas as a diffuse pattern, (Fig. 3A, 3B). In carcinomas, it was stained strongly as a diffuse pattern (Fig. 3C). In colorectal adenomas, the positive rate of expression of EGFR was 24%, and in colorectal carcinomas, it was 60%; this was a statistically significant difference (P < 0.05). In regard to the expression level of EGFR according to the histological dysplasia of the colorectal adenoma and according to the tumor size, similar to COX-2, no significant differences were observed (Table 3). Among adenomas, the positive rate in high grade dysplasia cases was 50%, and that in low grade dysplasia cases was 15.8%. Among low grade dysplasia cases, according to tumor size, the positive rate in cases with tumors larger than 5 mm was 21.7%, and that in cases with tumor sizes smaller than 5 mm was 7%.
In colorectal adenomas and carcinomas, the expression of COX-2 was related with that of VEGF, and that relation was statistically significant (Table 4).
The relation of the expression of COX-2 with EGFR was statistically significant in adenomas. Nonetheless, in carcinomas, with or without the expression of COX-2, COX-2 expression agreed with EGFR expression in 65% of the cases (26/40), but this difference was not statistically significant (Table 5).
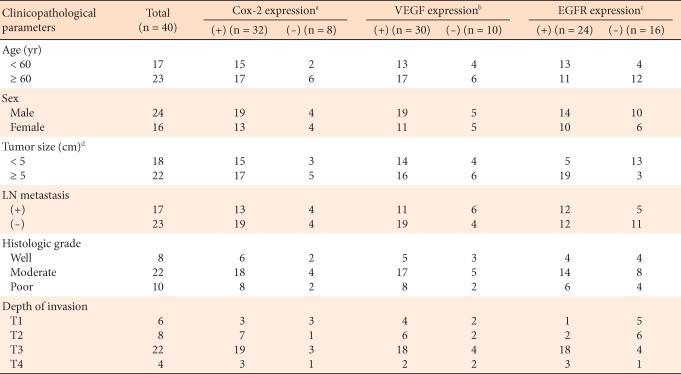
In colorectal carcinoma, no statistically significant differences were noted in the analysis of the correlations of the expressions of COX-2 and VEGF to age, gender, tumor size, the existence or absence lymph node metastasis, histological differentiation grade, or pathological stage, although the expression rate was high in elderly males, cases with tumors larger than 5 cm, cases with lymph node metastasis, and cases with tumors higher than moderate histological grade. The expression of EGFR was shown to be statistically significantly higher in tumors with sizes larger than 5 cm and in cases with lymph node metastasis. No significant correlations to other clinicopathological factors were noted (Table 6).
With the change to a westernized diet, colorectal cancer has become one of cancers rapidly increasing in Korea. Since COX-2 has been suggested to be closely associated with the development of colorectal cancer, a very important task is to elucidate differences between the expressions of COX in colorectal adenomas and carcinomas, the roles of those differences during the tumor progression process, and their association with prognostic factors.
COX-2 has been shown not to be expressed in the large intestinal mucosa, but its expression is increased in colorectal cancer [19]. In colorectal cancer, the expression of COX-2 in epithelial cells has been reported to alter their cohesiveness and to render them resistant to apoptosis, thus accelerating tumor growth and infiltration [20]. The precise role of COX-2 in the oncogenic course has not yet been elucidated; nevertheless, Tsujii and DuBois [20] observed that when rat intestinal epithelium was stably transfected with a COX-2 expressing vector, the cohesiveness of the cells to the extracellular matrix was increased, and resistance to natural death was increased; thus, they reported that such processes were closely associated with the development of colorectal cancer. In addition, it was proven that such changes could be reverted to the original condition by COX-2 selective inhibitors. Also, COX-2 has been reported to be involved in angiogenesis factors, such as VEGF and the neovascularization of tumor cells, and such changes could also be prevented by COX-2 selective inhibitors [21, 22]. Reviewing the reports on COX-2 in colorectal tumors, first, the expression rate of COX-2 in colorectal cancer has been reported to be 85-100% [19, 23, 24]. In colorectal adenomas and colorectal cancer, the expression of COX-2 has been reported to be elevated in proportion to the size of the tumor, and in colorectal sporadic adenomas, when the lesion was larger than 5 mm, the expression of COX-2 was significantly increased [25, 26]. Uefuji et al. [27] reported that in the analysis of COX-2 mRNA in gastric adenomas and gastric carcinomas, the expression of COX-2 correlated to the depth of tumor infiltration, the tumor size, and lymph node metastasis. It has been suggested that COX-2 induces tumor infiltration or metastasis through the COX-dependent PGE2 synthesis process and that it could suppress local tumor immunity. In our study, although it was not statistically significant, in colorectal adenomas, the expression rate of COX-2 in high grade dysplasia cases (66.7%) was higher than it was in low grade dysplasia cases (34.2%), and in low grade dysplasia adenomas larger than 5 mm, the expression rate was higher than it was in low grade dysplasia adenomas smaller than 5 mm (43.4% vs. 20%). However, a statistically significant high expression rate was shown in carcinomas in comparison with adenomas (80% vs. 44%). Although the number of cases was not large, this result is thought to support COX-2 beung induced in the early developmental stage in colorectal tumors. Such results of the association of the size of he adenoma with dysplasia are almost in agreement with previous studies [25, 26, 28]; nonetheless, it has been also reported that differences according to histological dysplasia have not been detected [29].
The expression of VEGF was similar to the pattern for the expression of COX-2. It was shown to be high in high grade adenomas larger than 5 mm, it was expressed at a higher rate in carcinomaa, and a significant difference was shown. This suggests the possibility that the induction of angiogenic factors and neovascularization are involved from the early developmental stage. In addition, in our study, in colorectal adenomas and carcinomas, the expression rate of COX-2 was proven to be correlated with the expression rate of VEGF, which result supports the role of COX-2 reported in other studies; i.e., it contributes to the vascularization within cancer by inducing the proliferation of vascular endothelial cells and controlling the blood flow within tumors. Nevertheless, in other studies, when COX-2 is overexpressed, a tendency of increased lymph node metastasis and early recurrence has been reported [12, 24]. In our study, in colorectal cancer, no association of the expression of COX-2 or VEGF with other clinical and pathological factors (age, gender, tumor size, presence or absence lymph node metastasis, histological differentiation grade, and pathological stage) was detected. With the accumulation of more cases, the further analysis related to carcinoma site and the histological type of the adenoma should be considered.
EGFR has drawn attention as a factor involved in the progression of cancer and metastasis, and it has been known to be involved in dysplasia from the relatively early phase [15, 30]. In our study, the expression of EGFR was similar to the expression of COX-2 and VEGF; nonetheless, in colorectal cancer, no correlation of the expression of COX-2 to EGFR could be detected. Although the expression of COX-2 agreed with the expression of EGFR in 65% of the cases, this agreement was not statistically significant. This may be due to the fact that in regard to the expression of EGFR, in addition to PGE2, various other factors, such as h-catenin, activator, protein-1, early growth response-1 gene, interferon-regulatory factor-1, p53, etc., may be involved [30], and the number of subjects was small. However, in colorectal adenomas, the expression of COX-2 was shown to correlate to the expression of EGFR, which may suggest the possibility of a mutual interaction between the two factors in the relatively early phase of tumor development. In addition, in colorectal adenomas, when tumor size was larger than 5 cm, the expression of EGFR was high, which suggests the possibility EGFR being a prognostic factor.
In our study, in colorectal adenomas, the expression of COX-2 was associated with the level of histological dysplasia and tumor size, and in the early phase of tumor development and the growth stage, the induction of COX-2 may be associated with the progression of the tumor. The correlation of the expression of VEGF to the expression of EGFR suggests the possibility that besides tumor development and growth, COX-2 may be involved in the cancer's acquiring an infiltrative, as well as metastatic, potential through the interaction of other oncogenic factors. Therefore, in the future, as prophylactic chemotherapies become more important in colorectal cancer, studies on prophylactic chemotherapy using drugs selectively suppressing COX-2 and other related oncogenic factors and on the role of COX-2 as a prognostic factor should continue to be conducted with more cases.
References
1. Pinto S, Gallo O, Dilaghi M, Gallina E, Giannini A, Coppo M, et al. Prostaglandins in squamous cell carcinoma of the larynx: tumor and peritumor synthesis. Prostaglandins Leukot Essent Fatty Acids 1990;39:53ŌĆō57. PMID: 2339137.


2. Scioscia KA, Snyderman CH, Rueger R, Reddy J, D'Amico F, Comsa S, et al. Role of arachidonic acid metabolites in tumor growth inhibition by nonsteroidal antiinflammatory drugs. Am J Otolaryngol 1997;18:1ŌĆō8. PMID: 9006670.


3. Ondrey FG. Arachidonic acid metabolism: a primer for head and neck surgeons. Head Neck 1998;20:334ŌĆō349. PMID: 9588707.


4. Smith WL, DeWitt DL. Biochemistry of prostaglandin endoperoxide H synthase-1 and synthase-2 and their differential susceptibility to nonsteroidal anti-inflammatory drugs. Semin Nephrol 1995;15:179ŌĆō194. PMID: 7631045.

5. Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis, part II. J Natl Cancer Inst 1998;90:1609ŌĆō1620. PMID: 9811310.



6. Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 1997;99:2254ŌĆō2259. PMID: 9151799.



7. Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol 1997;39:1ŌĆō20. PMID: 9160111.


9. Ondrey FG, Juhn SK, Adams GL. Inhibition of head and neck tumor cell growth with arachidonic acid metabolism inhibition. Laryngoscope 1996;106:129ŌĆō134. PMID: 8583839.


10. Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, et al. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol 1998;274:G1061ŌĆōG1067. PMID: 9696706.


11. Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett 2010;295:7ŌĆō16. PMID: 20381235.


12. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997;94:3336ŌĆō3340. PMID: 9096394.



13. Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer: implications for tumor angiogenesis and metastasis. Neoplasia 2001;3:53ŌĆō61. PMID: 11326316.



14. Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 1993;12:303ŌĆō324. PMID: 8281615.


15. Dougherty U, Sehdev A, Cerda S, Mustafi R, Little N, Yuan W, et al. Epidermal growth factor receptor controls flat dysplastic aberrant crypt foci development and colon cancer progression in the rat azoxymethane model. Clin Cancer Res 2008;14:2253ŌĆō2262. PMID: 18413814.


16. Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993;53:4727ŌĆō4735. PMID: 8402650.

17. Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995;26:86ŌĆō91. PMID: 7821921.


18. Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum Pathol 2002;33:708ŌĆō714. PMID: 12196922.


19. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994;107:1183ŌĆō1188. PMID: 7926468.


20. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995;83:493ŌĆō501. PMID: 8521479.


21. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998;93:705ŌĆō716. PMID: 9630216.


22. Cianchi F, Cortesini C, Fantappi├© O, Messerini L, Schiavone N, Vannacci A, et al. Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am J Pathol 2003;162:793ŌĆō801. PMID: 12598314.



23. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1995;55:3785ŌĆō3789. PMID: 7641194.

24. Fujita T, Matsui M, Takaku K, Uetake H, Ichikawa W, Taketo MM, et al. Size- and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res 1998;58:4823ŌĆō4826. PMID: 9809985.

25. Hasegawa K, Ichikawa W, Fujita T, Ohno R, Okusa T, Yoshinaga K, et al. Expression of cyclooxygenase-2 (COX-2) mRNA in human colorectal adenomas. Eur J Cancer 2001;37:1469ŌĆō1474. PMID: 11506952.


26. Elder DJ, Baker JA, Banu NA, Moorghen M, Paraskeva C. Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression. J Pathol 2002;198:428ŌĆō434. PMID: 12434411.


27. Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol 2001;76:26ŌĆō30. PMID: 11223821.


28. Sato T, Yoshinaga K, Okabe S, Okawa T, Higuchi T, Enomoto M, et al. Cyclooxygenase-2 expression and its relationship with proliferation of colorectal adenomas. Jpn J Clin Oncol 2003;33:631ŌĆō635. PMID: 14769841.



29. Einspahr JG, Krouse RS, Yochim JM, Danenberg PV, Danenberg KD, Bhattacharyya AK, et al. Association between Cyclooxygenase expression and colorectal adenoma characteristics. Cancer Res 2003;63:3891ŌĆō3893. PMID: 12873979.

30. Cohen G, Mustafi R, Chumsangsri A, Little N, Nathanson J, Cerda S, et al. Epidermal growth factor receptor signaling is up-regulated in human colonic aberrant crypt foci. Cancer Res 2006;66:5656ŌĆō5664. PMID: 16740703.


Fig.┬Ā1
(A) Cyclooxygenase-2 (COX-2) expression in the dysplastic epithelium as weak and diffuse (immunohistochemistry, ├Ś 100). (B) COX-2 expression in an adenocarcinoma as strong and diffuse (immunohistochemistry, ├Ś 100).
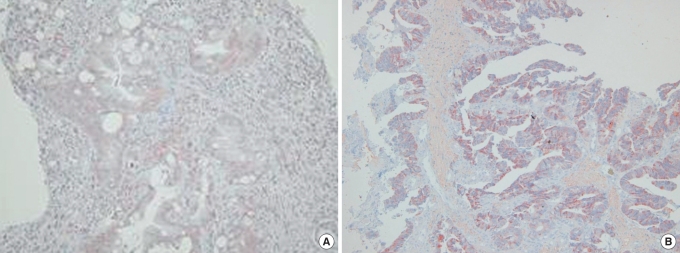
Fig.┬Ā2
(A) Vascular endothelial growth factor (VEGF) expression in the dysplastic epithelium as moderate and diffuse (immunohistochemistry). (B) VEGF expression in an adenocarcinoma as strong and focal (immunohistochemistry, ├Ś 100).
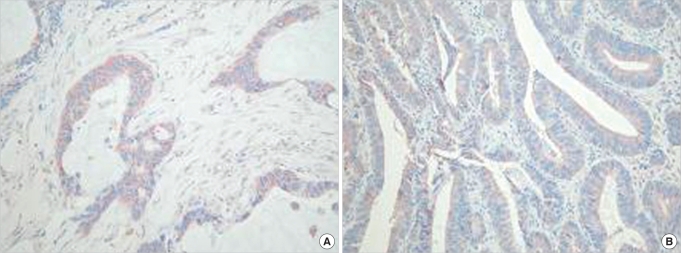
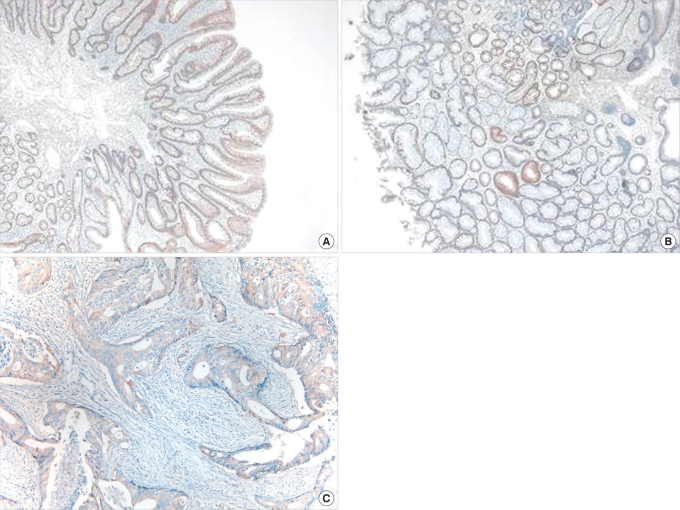
Fig.┬Ā3
(A) Mild diffuse expression of endothelial growth factor receptor (EGFR) in an adenoma (immunohistochemistry, ├Ś 40). (B) Aberrant expression of EGFR in an adenoma (immunohistochemistry, ├Ś 40). (C) Increased expression of EGFR in an adenocarcinoma (immunohistochemistry, ├Ś 100).