INTRODUCTION
A secure tension-free anastomosis is of paramount importance for successful enterocolic surgery, and the hand-sewn or stapling technique is currently the most popular technical standard for restoration of bowel continuity. However, both techniques inevitably leave foreign materials within tissue, evoking an inflammatory reaction, thus potentially resulting in a number of anastomosis-related adverse effects, including bleeding, leakage, and stricture. These drawbacks associated with conventional anastomotic techniques naturally lead surgeons to turn their attentions to sutureless methods of anastomosis.
As one of the sutureless techniques, the concept of compression anastomosis has long been investigated. As well as being safe enough to be air-tight, the ideal device for compression anastomosis should be easy and quick to manipulate and leave a wide and patent orifice for the anastomosis. In 1985, the biofragmentable anastomotic ring (Valtrac BAR, Covidien, Mansfield, MA, USA) was developed by Hardy et al. [1], and a number of studies confirmed that the Valtrac BAR was a safe and reliable compression anastomotic device in both elective and emergency surgery [2-5]. In the new millennium, a novel compression anastomosis clip (Hand CAC 30, NiTi, Surgical Solutions Ltd., Netanya, Israel) was introduced. It is made of a shape-memory alloy of nickel-titanium (Nitinol), which is temperature dependent. Because this device is staple-free, there is no puncturing injury of the bowel wall, no risk of anastomotic bleeding, and no permanent foreign material left within the body after healing. Within 7 to 10 days, the clip, together with the necrotized tissue, detaches and is naturally eliminated from the body during bowel movements, leaving a wide and patent sutureless anstomosis [6-8]. The aims of the current study were to present early clinical experience with the Hand CAC 30 in enterocolic side-to-side anastomosis after diverse colonic or enteric resections and to evaluate the safety, efficacy, and technical feasibility of the device.
METHODS
Patients
A non-randomized prospective data collection was performed for patients undergoing a colonic or enteric resection followed by a side-to-side anastomosis using the Hand CAC 30 for various etiologies. Eligibility criteria for the use of the Hand CAC 30 were anastomoses between the colon and the ileum (enterocolic) or between the two small bowels (enteroenteric). For colonic resection, bowel preparation was performed on the day before surgery and consisted of mechanical whole gut irrigation with four liters of polyethylene glycol solution (Colonlyte, Dream Pharma Co., Seoul, Korea) combined with oral metronidazole and additional prophylaxis with parenteral metronidazole just before surgery. The surgical procedures were performed by a single surgeon (HJC). Between November 2009 and February 2011, a total of 63 anastomoses were constructed by use of the Hand CAC 30. Preoperatively, all patients were informed of various methods of bowel anastomosis, and the anastomosis was constructed using the Hand CAC 30 under their consents. Data on patient demographics, surgical indications, operative procedure, perioperative course, and outcome were recorded prospectively on data sheets. The primary short-term clinical endpoint was the rate of anastomotic leakage, and other clinical outcomes, including intra- and postoperative complications, lengths of operation time and hospital stay, and the clip elimination time, were recorded. No postoperative radiologic contrast study was performed routinely, and anastomotic leakage was diagnosed clinically.
Description and use of the Hand CAC 30
Nitinol is a temperature-dependent shape memory alloy of nickel-titanium because it expands and flexes when cooled, but resumes its original shape and size when it returns to its normal temperature [9]. When the device is cooled in cold saline for over 30 seconds, it becomes supple with an opening angle of 30┬░ [7, 8]. When an anastomosis is created, the two closed rings can accommodate to different thicknesses of tissue within the anastomosis [6, 7]. At body temperature, it returns to its original shape, which closes the gap gradually until the trapped tissue becomes necrotic, and healthy tissue connects the bowel walls at the outer perimeter of the ring, thus restoring bowel continuity [6-8].
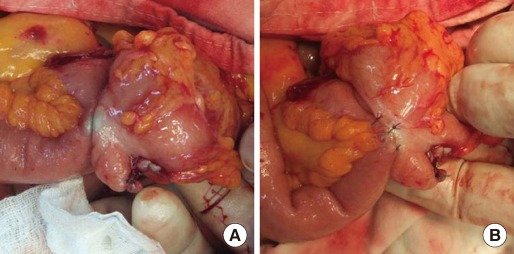
The Hand CAC 30 instrument consists of three main components, an applier body, a nickel-titanium clip, and clip loader (Fig. 1). The anastomosis clip made of nickel-titanium alloy is an elliptically-shaped double-ring device with a diameter of 30 mm. Its use is quite simple, and it was manipulated according to the manufacturer's instructions. In brief, after completing the resection of the diseased bowel by using a linear stapler at the proximal and the distal ends, we prepared each bowel segment for anastomosis as follows. The two edges of the bowels to be anastomosed were placed parallel to each other in an iso-peristaltic direction overlapping a minimum length of 5 cm. After the two edges had been secured by placing an anchoring stitch between the bowels at the anti-mesenteric walls, one stitch at each side of the overlap, a parallel 5-mm-long enterotomy (or colostomy) incision was made at the anti-mesenteric border of each segment, and the inside ends of the incisions were secured to each other by using 4-0 absorbable sutures. Simultaneously, the Hand CAC 30 device was prepared as follows before use: After the loader had been pushed towards the applier to mount the clip on the jaw of the applier, the loader was removed, and the clip mounted on the applier was immersed in ice-cold saline for at least 30 seconds. For anastomosis, the lever was pressed (1st press) all the way down to open the jaw of the applier, and each ring of the clip was introduced all the way into each bowel lumen through the incisions. The lever was pressed again (2nd press) to close the clip and clamp the tissues. After this position had been retained for about 20 seconds to enable complete compression, the lever was pressed again (3rd press) to create a slit in the clamped tissues by using a specially incorporated blade in the applier. The lever of the applier was pressed for the last time (4th press) to release the clip from the applier. After the applier had been removed and the clamped clip and the slit had been secured on the tissue, the two incisions were closed in two layers. After closure of the enterotomy (or enterocolototomy), we also reinforced the opposite sides of the anastomosed walls by using several stitches because the sides of the anastomosis walls were always thinned by the compressed clip (Fig. 2).
RESULTS
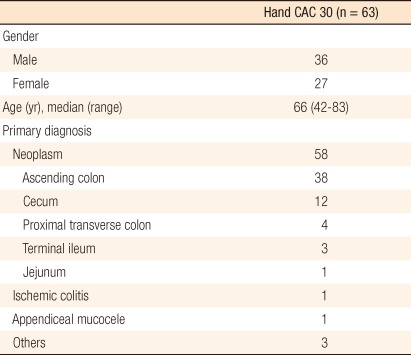
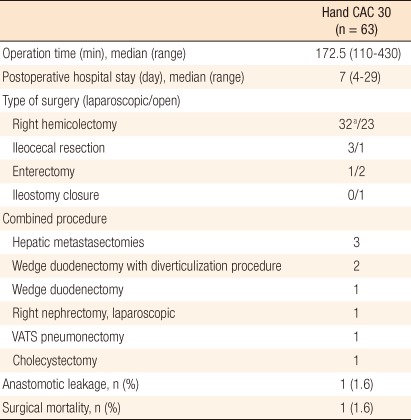
A total of 63 patients (male, 36) underwent an enteric or right-sided colonic resection followed by a side-to-side anastomosis using the Hand CAC 30. Patient demographics and primary diagnoses for an intestinal resection are shown in Table 1. Patients ranged in age from 42 to 83 years (median, 66 years). A majority of the patients (92%) received colonic or enteric surgery for neoplastic etiologies. Surgical and pathologic data are shown in Table 2. The median lengths of the operation time and the postoperative hospital stay were 172.5 minutes and 7 days, respectively. Laparoscopic surgery was performed in 36 patients, in whom one patient who underwent a laparoscopic right hemicolectomy for right-sided colon cancer was converted to an open procedure (1/32, 3.1%) for bleeding from injury of the Henle's trunk. Combined procedures were performed in 9 patients (3 hepatic metastasectomies, 2 wedge duodenectomies with a diverticulization procedure, 1 wedge duodenectomy, 1 laparoscopic right nephrectomy, 1 video-assisted pneumonectomy, 1 cholecystectomy).
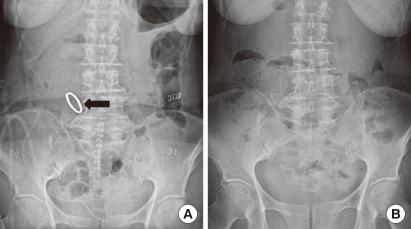
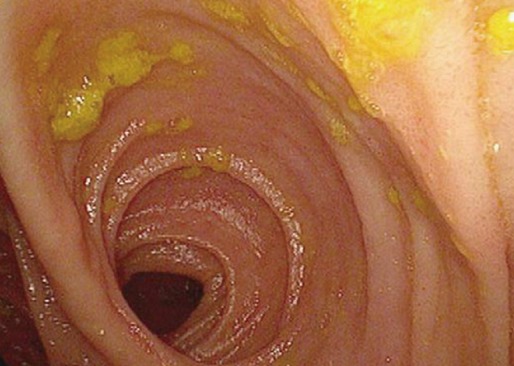
No complications associated with the device occurred during the operation, and no premature postoperative dislodgement of the clip was observed in any of the patients (Fig. 3A). One patient with ascending colon cancer showed anastomotic leakage after 5 days postoperatively. This 80-year-old female received combined open right hemicolectomy for a huge ascending colon cancer (poorly differentiated neuroendocrine carcinoma) and cholecystectomy for Mirizzi syndrome. A second exploration showed disruption of the enterocolotomy site, and primary suture closure of the disruption (with the clip left) and a temporary diverting ileostomy were performed. She did not recover and died of co-morbid ischemic heart disease after 24 days postoperatively. There were no other surgical mortalities. All patients were informed that the clip would be expelled with bowel movement within 14 days after surgery. Practically, exact date of expulsion of the clip could not be recorded because most patients were not aware that the clip had been evacuated with the stool. Evacuation of the clip was confirmed by plain abdominal radiographs during outpatient follow-up, and no patient was found to have retained the clip within the bowel (Fig. 3B). Postoperatively, no patient manifested clinical symptoms, suggestive of anastomotic stricture. Colonoscopy at the 6-month follow-up showed a functioning wide anastomois constructed by the Hand CAC 30 (Fig. 4).
DISCUSSION
The basic concept of compression anastomosis is that two bowel ends are compressed together by a sutureless device, preventing leakage and facilitating the natural healing in the compressed region. Theoretically, compression anastomosis is thought to be an ideal method of anastomosis because it leaves no permanent foreign material at the anastomosis and secures serosa-to-serosa approximation of the anastomosis. The history of the development of compression anastomotic devices goes back to the 19th century. After metal rings devised by Denan in 1826 and "Murphy's button" in 1892 [10], another 100 years passed before a new compression anastomosis device was put into wide clinical use. In 1985, Hardy et al. [1] developed a biofragmentable anastomosis ring (Valtrac BAR) made of absorbable polyglycolic acid and radiopaque barium sulfate. For the prevention of tissue ischemia and the accommodation of tissue thickness, the margins of two identical rings are scalloped in shape and three gap-widths in the closed position (1.5, 2.0, and 2.5 mm) are available; however, several intraoperative problems have been reported, including failure of the purse-string sutures, inappropriate selection of the size of the Valtrac BAR with partial or full-thickness tears of the bowel wall, and failure of the snap or shattering of the device [4, 5].
Beginning with the new millennium, a novel compression anastomotic device, the Hand CAC 30, was introduced. Although the basic mechanism of this device composed of a nickel-titanium alloy is not different from that of Murphy's button or the Valtrac BAR, it has unique physical properties: temperature-dependent shape memory and super-elasticity [7]. The safety of this device has been demonstrated in animal studies, and the resected segments showed a complete epithelial lining throughout the anastomosis, indicating an intact, uniform, and well-functioning anastomosis [6]. In comparison to other compression devices, distinctive theoretical merits of the Hand CAC 30 are that the flexibility of the device at low temperatures can allow it to accommodate different thicknesses of the anastomosis and that it provides a constant, continuous, and uniform pressure over the clamped tissue along its compressed perimeter [11]. The Hand CAC 30 was approved by the US Food and Drug Administration (FDA) for use in intestinal anastomoses in September 2004 and by the Korea FDA in April 2008.
Clinical experiences with the use of the Hand CAC 30 in enteric or colonic anastomoses are still scarce. A prospective single-center study comparing colonic anastomosis using the Hand CAC 30 (30 patients) with stapled anastomosis (30 patients) reported that neither group had anastomotic complications such as leakage or obstruction and that parameters such as hospital stay, start of gases and bowel movement, cessation of antibiotic treatment, and removal of catheter were better in the study group than in the control group [7]. A smaller prospective study comparing Hand CAC 30 anastomosis (5 patients) with stapled anastomosis (5 patients) in laparoscopic colonic surgery also showed that no complications associated with the device occurred during the postoperative period in any of the patients [12]. In line with the promising conclusion in colonic anastomosis, two randomized control trials evaluating the clinical validities of the Hand CAC 30 in small-intestinal anastomosis have been published recently. A Chinese study performed gastrointestinal anastomosis proximal to the ileocecal valve by using the Hand CAC 30 in 33 patients and compared their results to those of another 33 patients in which a stapled anastomosis had been constructed [13]. The anastomosis was fashioned to reconstruct a Roux-en-Y loop, enteroentrostomy, Billroth II gastrojejunostomy, and gastrojejunostomy. They found no postoperative complication related to the anastomotic method in either group and concluded that the Hand CAC 30 could be used not only in colonic surgery but also in gastrointestinal anastomosis. The most recent multicenter prospective randomized trial in Korea was conducted to determine the clinical efficacy of the Hand CAC 30 for a jejunojejunostomy in gastric-cancer surgery [14]. Patients with a gastric carcinoma were randomized into a CAC group (20 patients) and a hand-sewn group (24 patients) for a Roux-en-Y jejunojejunostomy after a total gastrectomy. Two patients in the Hand CAC 30 group experienced leakage. The authors inferred that these leaks might reflect a learning curve because they occurred in the earlier period of the trial, and the authors emphasized the importance of complete mastery of the use of the devices before clinical application.
Although not randomized in design, the current study involves the largest sample size ever presented and demonstrates the clinical safety relevant to Hand CAC 30 anastomosis in both laparoscopic and open surgeries for diverse clinical indications. In the current study, only one patient with ascending colon cancer showed anastomotic leakage after 5 days postoperatively. For over 20 years, the Valtrac BAR, rather than staplers or suture materials, has been the routine device in open ileocolic, ileorectal, colorectal, or colocolic anastomosis in our practice. Our collective data of 632 anastomoses using the Valtrac BAR showed a leakage rate of 0.8% [15]. The overall rate of anastomotic leakage after a right colectomy (both stapled and hand-sewn anastomoses) in recent data was between 1.35% and 2% [16, 17]. Compared to these results, the leakage in one patient (1.6%) in this study implies that the Hand CAC 30 anastomosis is as safe and efficacious as a conventional hand-sewn or stapling anastomosis.
Until today, the most popular mechanical device for construction of an anastomosis in colorectal surgery has been the linear stapler or circular stapler. As emphasized by Nudelman et al. [7], the Hand CAC 30 has several advantages over the stapler. First, the cut raw surface at the edge of the stapler line can lead to stricture. However, the cut surface of the Hand CAC 30 anastomosis is already covered by mucosa, leaving a smooth and perfect wall. Second, the anastomosis using the Hand CAC 30 is much wider because the established anastomosis takes the size of the outer perimeter, not the inner perimeter as with the stapler. Another merit of the Hand CAC 30 is that the absence of permanent foreign bodies may greatly decrease inflammatory stimuli and formation of fibrous tissue. These superiorities in biologic healing should be advantageous in the prevention of anastomotic stenosis. In addition, anastomosis using the Hand CAC 30 is financially superior to other methods of anastomosis. Apart from other cost factors associated with surgery, the Hand CAC 30 itself costs about 200,000 Korean Won (KRW, about 180 $), which is much more cost-effective than an end-to-end stapling device (390,000 KRW) or a GIA stapler (about 390,000 KRW or more).
Issues associated with the CAC anastomosis deserve mentioning. Although the Hand CAC 30 is a compression anastomotic device, a small incision in the bowel walls is essential to insert the device, and suture closure is necessary after the device is deployed. This suture closure may be a source of leakage, and the anastomotic leakage in this study was due to failure of the suture closure. A meticulous, standard, two-layered closure should be done for a more secure completion of the anastomosis. The other issue the authors would like to mention is that the opposite sides of the insertion incisions should be inspected after the suture closure. In the authors' experience, the sides of the anastomosis were always thinned by the compressed clip (Fig. 2A). To prevent compression necrosis of the bowel wall, the authors would like to recommend reinforcing the anastomosed walls to each other by using several stitches (Fig. 2B). Although there are no contraindications for the Hand CAC 30 anastomosis in enterocolic surgery, clinical data on the safety of the Hand CAC 30 anastomosis to date are restricted to elective enterocolc surgery. Further prospective large-scale studies might be imperative to determine if a Hand CAC 30 anastomosis can be constructed safely in a diseased bowel as in Crohn's disease and if it can be performed during emergency surgery.
In conclusion, short-term evaluation of the Hand CAC 30 anastomosis in patients undergoing laparoscopic or open enterocolic surgery proved to be a safe and efficacious alternative to the standard hand-sewn or stapling technique. Along with its technical superiorities, a prospective large-scale clinical trial would confirm the validity of the Hand CAC 30 in diverse clinical settings.