- Search
|
|
Abstract
Purpose
Although the height of a rectal tumor above the anal verge (tumor height) partly determines the treatment strategy, no practical standard exists for reporting this. We aimed to demonstrate the differences in tumor height according to the diagnostic modality used for its measurement.
Methods
We identified 100 patients with rectal cancers located within 15 cm of the anal verge who had recorded tumor heights measured by using magnetic resonance imaging (MRI), colonoscopy, and digital rectal examination (DRE). Tumor height measured by using MRI was compared with those measured by using DRE and colonoscopy to assess reporting inconsistencies. Factors associated with differences in tumor height among the modalities were also evaluated.
Results
The mean tumor heights were 77.8 ┬▒ 3.3, 52.9 ┬▒ 2.3, and 68.9 ┬▒ 3.1 mm when measured by using MRI, DRE, and colonoscopy, respectively (P < 0.001). Agreement among the 3 modalities in terms of tumor sublocation within the rectum was found in only 39% of the patients. In the univariate and the multivariate analyses, clinical stage showed a possible association with concordance among modalities, but age, sex, and luminal location of the tumor were not associated with differences among modalities.
The height of a rectal tumor above the anal verge (tumor height) is an important factor in determining the treatment planŌĆöfor example, administering preoperative chemoradiotherapy (PCRT) or sphincter preservationŌĆöand for comparing the results of treatment. Several studies have reported differences in oncologic outcomes (local recurrence or disease-specific survival) according to tumor height [1-4]. In addition, tumor height is suggested as a standard for determining which surgical treatment, such as transanal excision, is most appropriate [5-7].
The method used to measure tumor height varies across institutions and among studies; there is no consensus on a standard method. Moreover, the method used is often not stated in rectal cancer studies. In some trials, a rigid proctoscope was used to measure tumor height; in another phase II study, digital rectal examination (DRE) and colonoscopy were used [8-10]. Some prospective trials did not describe the method used [11, 12]. Similarly, heterogeneity of measurement modalities is also found in several comparative studies on low rectal cancers [13-17]. However, tumor height can differ according to the modality used for measurement, even in the same patient; this can result in confusion regarding which value to use to make treatment decisions.
Unfortunately, there is a paucity of evidence regarding the extent of the measurement differences among diagnostic modalities and regarding which method is most accurate. Hence, this study aimed to compare the tumor height, as measured by using magnetic resonance imaging (MRI), DRE and colonoscopyŌĆöall commonly used in diagnosing rectal cancerŌĆöand to evaluate factors associated with differences among these diagnostic modalities.
From March 2014 to November 2016, we identified 100 consecutive patients with rectal cancer located within 15 cm of the anal verge for whom recorded tumor height values, measured by using MRI, DRE, and colonoscopy, at Asan Medical Center, Seoul, Korea, were recorded. This study was approved by the Asan Medical Center Institutional Review Board (S2018-0464-0001), and was eligible for exemption from informed consent. Tumor height was measured before patients underwent PCRT because the measurement might be different after PCRT due to tumor regression and because in patients with a good response to PCRT, determining the tumor height via DRE might be difficult. Patients who did not undergo at least one of these diagnostic modalities were excluded.
DRE was performed with the patient in a left lateral decubitus position. After lubrication had been applied, the examinerŌĆÖs gloved index finger was gently inserted up to the rectal cancer; the tumor height was measured from the anal verge to the tip of the straightened finger. Four expert colorectal surgeons performed the DREs; each of them had experience with treating at least 500 patients with rectal cancer at a single institution. Colonoscopy was performed by endoscopic specialists with a flexible colonoscope (CF-H260 AL/I, CF-HQ290 AL/I, Olympus, Tokyo, Japan). On withdrawal of the scope, tumor height was measured from the lower margin of the tumor to the anal verge.
An expert, board-certified, gastrointestinal radiologist, who had reviewed the MRI images of 300 patients with rectal cancer, reviewed the MRI images of the study subjects to determine the tumor length and the anatomical distance from the anal verge to the lowest margin of the tumor, from the anal verge to the upper margin of the tumor, and from the anorectal junction to the lowest margin of the tumor. In accordance with a national recommendation, tumor height was measured by using a curvilinear measurement and by drawing multiple linear lines along the approximate luminal center of the rectum and the anus in the midline sagittal plane (Fig. 1) [18].
We divided rectum into 3 parts, as defined in the chapter on the surgical treatment of patients with rectal cancer in the American Society of Colon and Rectal SurgeonsŌĆÖ textbook on colon and rectal surgery, as follows: lower rectum, <5 cm; midrectum, 5ŌĆō10 cm; and upper rectum, 10ŌĆō15 cm from the anal verge [6]. In addition, we classified tumors according to their intraluminal location, as determined by MRI, as ventral, lateral, dorsal, or encircled masses.
Categorical variables were compared using Pearson chi-square test. Continuous variables were compared using the independent samples t-test and by computing intraclass correlation coefficients. Logistic regression was used for univariate and multivariate analyses, and both Cohen kappa and weighted kappa tests were performed to evaluate agreement in the measurements of tumor height among modalities. Univariate and multivariate analyses were used to identify factors associated with concordance of tumor height measurements among modalities. All numbers are expressed as means and standard deviations. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
The median age of the patients was 59 years, with a male preponderance (60% men). Using MRI, we found that the mean longitudinal tumor length was 36.7 ┬▒ 1.3 mm and that most tumors were located laterally in the intestinal lumen. Furthermore, most tumors (64%) were clinical T3 stage, and 80% were determined to have a positive nodal status. Patients underwent sphincter-saving surgery, abdominoperineal resection, or transanal excision according to their cancer stage and location; those who met the criteria underwent surgery after having received PCRT. Pathologically, the majority of patients had stage T3 tumors (50%) and a negative nodal status (54%). The rates of positive lymphovascular and perineural invasion were 25% and 16%, respectively (Table 1).
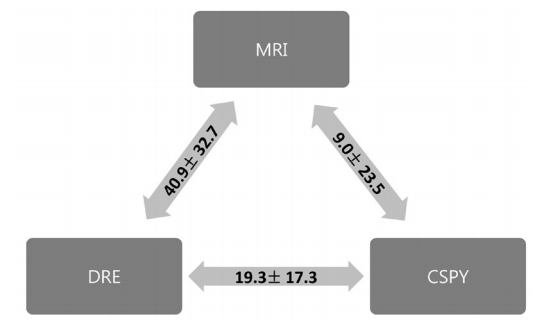
The mean heights of the low margin of the tumor above the anal verge were 77.8 ┬▒ 3.3, 52.9 ┬▒ 2.3, and 68.9 ┬▒ 3.1 mm as measured by using MRI, DRE, and colonoscopy, respectively (P < 0.001). The mean difference was largest between measurements obtained via MRI and DRE (40.9 ┬▒ 32.7 mm), and was smallest between those obtained via MRI and colonoscopy (9.0 ┬▒ 23.5 mm). The mean difference between DRE and colonoscopy was 19.3 ┬▒ 17.3 mm (Fig. 2).
Most tumors were located in the midrectum according to MRI (57%) and colonoscopy (57%), but DRE reported most tumors (58%) as being low rectal cancers. The proportion of upper rectal cancers identified was greatest when assessed by using MRI rather than the other diagnostic modalities (MRI, 20%; DRE, 2%; colonoscopy, 7%; P < 0.001) (Table 2). The upper tumor margin was a mean distance of 118.2 ┬▒ 3.9 mm above the anal verge when measured by using MRI. Most midrectal cancers (21 of 35, 60%) extended into the upper rectum.
When the tumors were classified into their sublocation within the rectum, the locations of 39 tumors (39%) reported by the three modalities were the same. MRI showed 59% and 42% of concordance rates with colonoscopy and DRE. Between DRE and colonoscopy, the rate of concordance was 71%. Statistically, both DRE and colonoscopy showed mild to moderate concordance with MRI according to Cohen kappa and the weighted kappa analyses. In particular, MRI showed slightly more concordance with colonoscopy than with DRE (Table 3).
In the univariate analysis, early T stage (cT1 or 2) and negative nodal status were associated with concordance in the measurements of tumor height among the 3 modalities. In the multivariable analysis, only early cT stage was, although not significantly (P = 0.069) (Table 4).
In the present study, we observed notable variation in the measurement of tumor height among the three diagnostic modalities. Moreover, the concordance between the measurements obtained was unsatisfactory. Each diagnostic modality has its own characteristics. DRE is easy to perform and provides tumor fixity and the exact intraluminal location of the tumor. However, it is a subjective measure and cannot assess most upper rectal cancers; hence, the information obtained via DRE, compared with other modalities, is limited. Furthermore, it can miss early rectal cancers [6]. In this study, the values of tumor heights measured by using DRE were shorter than they were for the other modalities.
A rigid proctoscope allows for accurate measurement of the tumor height [19], but it has been replaced by the long-length flexible scope; the latter gives more information and examines the whole large intestine. Recently, most studies and hospitals, including our institution, have not used rigid scope for height measurements of rectal cancer. For this reason, this study adopted the flexible scope, which is the preferred diagnostic modality, rather than a rigid scope. Colonoscopy gives more information, including the overall shape, size, and intraluminal status of a rectal tumor. However, when using a flexible scope, the measurement of tumor height may be inaccurateŌĆömeasuring a distance greater than the true distanceŌĆödepending on how much of the rectum is redundant [7]. In this study, the concordance was higher between MRI and colonoscopy than between MRI and DRE. MRI and flexible colonoscopy use curved or curvilinear measurements, but DRE tends to measure a straight distance with a straightened finger. These different measurement mechanisms may be the reason for the higher concordance between MRI and colonoscopy.
MRI allows for objective measurement of the location of a rectal tumor. Additionally, it can provide information about the tumorŌĆÖs progression, its relationship with adjacent organs, and its overall shape. However, detecting early or small lesions by using MRI may be difficult, and sometimes DRE can establish more accurately than MRI whether the rectal cancer has invaded the vagina or prostate gland. However, MRI is more beneficial than DRE or colonoscopy in cases of obstructive rectal cancer. Each diagnostic modality has its own advantages and shortcomings, yet comparative studies are difficult to find. Thus, the feature of the rectal cancer must be used to determine which modality is most appropriate and should be used.
According to several reports, the measurement of tumor height may be affected by many possible factors, such as age, sex, tumor stage, luminal location, and the posture of the patient. The rectal length can be altered by changes in the tone of the anal sphincter complex that occur in the awake and the anesthetized states [20]. In addition, the external anal sphincter is reported to be shorter in women than in men and to become thinner with aging [21]. Some studies found that the level of peritoneal reflection differed between men and women [7, 22, 23], whereas others reported no sex-specific differences, reporting rather that the level of the sacral promontory or the patientŌĆÖs height was more important [24, 25]. In this study, age and sex did not affect agreement in the measurements of tumor height among the 3 modalities (age, P = 0.30; sex, P = 0.56). Contrary to age and sex, the fixity and the luminal location of the tumor do affect the measurement of tumor height by each modality.
We thought that the rate of concordance among the diagnostic modalities would be higher for cancers with a more advanced T-stage because we thought that the fixity of the tumor would provide a more constant tumor height than would be found in early cancers with redundant rectum. However, the rate of concordance was higher in early cancers (65%, 13 of 20) than advanced cancers (32.5%, 26 of 80). Earlier staged cancers do not show fixation to adjacent structures, so during a measurement such as with DRE or colonoscopy, the distal rectum would be more freely straightened than it would be for advanced cancers. This may be the reason for the difference in concordance rate according to tumor stage. Moreover, due to the curved anatomy of the rectum, we presumed that the luminal location of the rectal cancer would be associated with the measurement of tumor height by each modality; however, luminal tumors were not associated with the rate of concordance among the modalities (P = 0.393). Additional studies with larger pools of patients are needed to verify this finding.
In this study, 61.4% of midrectal tumors were located above the level of the peritoneal reflection and 75.4% extended into the upper rectum. Interestingly, in 1 patient (4.3%) with a low rectal cancer, the (large) tumor extended above the level of the peritoneal reflection. The peritoneal reflection and whole extent of the tumorŌĆöincluding its upper marginŌĆöare important landmarks for determining the type of treatment. In our institution, DRE was the most reliable modality for determining the length of the distal margin for low or midrectal cancers before radical surgery, and MRI was useful for comparing levels with adjacent structures, such as the peritoneal reflection, when a patient is scheduled for PCRT.
This study has some limitations. First, bias according to the test performers is possible. DREs were performed by 7 surgeons in this study, but no significant differences according to the surgeon were noted (P = 0.081) when DRE measurements were compared with MRI measurements. Colonoscopy was also performed by many specialists. However, due to the small number of enrolled patients, statistical analyses according to the colonoscopists were not possible. However, the tumor height from the anal verge was routinely checked during withdrawal of the scope with reduced curvature. Second, we only demonstrated differences among diagnostic modalities; we did not suggest which modality was an adequate tool for determining rectal cancer treatment. However, we can conclude that finding a standard best tool is not practical in this era of diverse treatment options; rather, a consensus on landmarks and more diversified indications are needed for the various treatment options.
In this era of diverse treatment options for patients with rectal cancer, the tumor height of the rectal cancer is an important variable for determining the treatment option and posttreatment oncologic outcomes. However, in this study, the concordance rate among three modalities (MRI, DRE, and colonoscopy) for measuring the tumor height was only 39%. Thus, well-designed studies are needed to establish more diversified indications according to the treatment options, as is more discussion on a descriptive consensus on how to measure the tumor height.
Fig.┬Ā1.
Tumor height measurement using magnetic resonance imaging. The location of the rectal cancer (red arrows) and the tumor height were measured from the anal verge to the lowest margin of the cancer along the luminal center of the anorectum in the midsagittal plane (red lines).
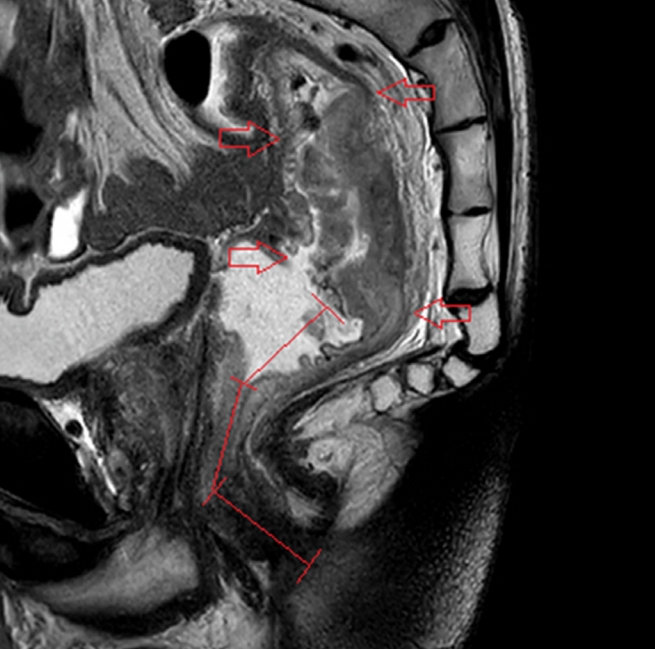
Fig.┬Ā2.
Difference in the height of the tumor among modalities (mean ┬▒ standard deviation, mm). The mean difference was the largest between MRI and DRE while the smallest was between MRI and CSPY. MRI, magnetic resonance imaging; DRE, digital rectal examination; CSPY, colonoscopy.
Table┬Ā1.
Characteristics of the patients (n = 100)
| Variable | Value |
|---|---|
| Age (yr) | 59.0 ┬▒ 13.1 |
| Sex | |
| ŌĆāMale | 60 (60) |
| ŌĆāFemale | 40 (40) |
| cT stagea | |
| ŌĆā1 | 1 (1) |
| ŌĆā2 | 19 (19) |
| ŌĆā3 | 64 (64) |
| ŌĆā4 | 16 (16) |
| cN stagea | |
| ŌĆāNegative | 20 (20) |
| ŌĆāPositive | 80 (80) |
| Luminal tumor location | |
| ŌĆāVentral | 29 (29) |
| ŌĆāLateral | 31 (31) |
| ŌĆāDorsal | 19 (19) |
| ŌĆāEncircling | 21 (21) |
| Type of operation | |
| ŌĆāSphincter-saving surgery | 93 (93) |
| ŌĆāAbdominoperineal resection | 2 (2) |
| ŌĆāLocal excision | 4 (4) |
| ŌĆāNo treatment | 1 (1) |
| (y)pT (n = 99) | |
| ŌĆā0 | 10 (10) |
| ŌĆāTis | 1 (1) |
| ŌĆā1 | 12 (12) |
| ŌĆā2 | 23 (23) |
| ŌĆā3 | 50 (50) |
| ŌĆā4 | 3 (3) |
| (y)pN (n = 95) | |
| ŌĆāNegative | 54 (54) |
| ŌĆāPositive | 41 (41) |
| Lymphovascular invasion (n = 99) | |
| ŌĆāNegative | 74 (74) |
| ŌĆāPositive | 25 (25) |
| Perineural invasion (n = 99) | |
| ŌĆāNegative | 83 (83) |
| ŌĆāPositive | 16 (16) |
Table┬Ā2.
Sublocation of rectal tumors according to diagnostic modality (n = 100)
| Modality and tumor location |
MRI |
Agreement rate | ||
|---|---|---|---|---|
| LR | MR | UR | ||
| DRE | ||||
| ŌĆāLR | 21 | 36 | 1 | |
| ŌĆāMR | 2 | 21 | 17 | 40% |
| ŌĆāUR | 0 | 0 | 2 | |
| Colonoscopy | ||||
| ŌĆāLR | 17 | 18 | 1 | |
| ŌĆāMR | 6 | 37 | 14 | 59% |
| ŌĆāUR | 0 | 2 | 5 | |
Table┬Ā3.
Concordance among MRI, colonoscopy, and DRE measurements of tumor height
|
DRE |
Colonoscopy |
|||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| Concordance | 0.44 | 0.34ŌĆō0.54 | 0.59 | 0.45ŌĆō0.65 |
| Cohen kappa | 0.12a | 0.07ŌĆō0.25 | 0.37a | 0.13ŌĆō0.45 |
| Weighted kappa | 0.25a | 0.14ŌĆō0.35 | 0.37a | 0.23ŌĆō0.51 |
Table┬Ā4.
Factors associated with MRI-based coincidence of tumor height
REFERENCES
1. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324ŌĆō32.


2. Marinello FG, Frasson M, Baguena G, Flor-Lorente B, Cervantes A, Rosello S, et al. Selective approach for upper rectal cancer treatment: total mesorectal excision and preoperative chemoradiation are seldom necessary. Dis Colon Rectum 2015;58:556ŌĆō65.


3. Pahlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjodahl R, et al. The Swedish rectal cancer registry. Br J Surg 2007;94:1285ŌĆō92.


4. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638ŌĆō46.


5. Gordon PH, Nivatvongs S. Principles and practice of surgery for the colon, rectum, and anus. 3rd ed. New York: Informa Healthcare; 2007.
6. Beck DE, Roberts PL, Saclarides TJ, Senagore AJ, Stamos MJ, Wexner SD. The ASCRS textbook of colon and rectal surgery. 2nd ed. New York: Springer; 2011.
7. Corman ML. CormanŌĆÖs colon and rectal surgery. 6th ed. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
8. Sauer R, Becker H, Hohenberger W, R├Čdel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731ŌĆō40.


9. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811ŌĆō20.



10. Roh MS, Colangelo LH, OŌĆÖConnell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124ŌĆō30.



11. Park JH, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer 2011;117:3703ŌĆō12.


12. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114ŌĆō23.


13. Klose J, Tarantino I, Kulu Y, Bruckner T, Trefz S, Schmidt T, et al. Sphincter-preserving surgery for low rectal cancer: do we overshoot the mark? J Gastrointest Surg 2017;21:885ŌĆō91.



14. Omidvari S, Hamedi SH, Mohammadianpanah M, Razzaghi S, Mosalaei A, Ahmadloo N, et al. Comparison of abdominoperineal resection and low anterior resection in lower and middle rectal cancer. J Egypt Natl Canc Inst 2013;25:151ŌĆō60.


15. Nakagoe T, Ishikawa H, Sawai T, Tsuji T, Tanaka K, Hidaka S, et al. Survival and recurrence after a sphincter-saving resection and abdominoperineal resection for adenocarcinoma of the rectum at or below the peritoneal reflection: a multivariate analysis. Surg Today 2004;34:32ŌĆō9.


16. Stelzner S, Hellmich G, Sims A, Kittner T, Puffer E, Zimmer J, et al. Long-term outcome of extralevator abdominoperineal excision (ELAPE) for low rectal cancer. Int J Colorectal Dis 2016;31:1729ŌĆō37.



17. Saito N, Sugito M, Ito M, Kobayashi A, Nishizawa Y, Yoneyama Y, et al. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg 2009;33:1750ŌĆō6.


18. KSAR Study Group for Rectal Cancer. Essential items for structured reporting of rectal cancer MRI: 2016 consensus recommendation from the Korean Society of Abdominal Radiology. Korean J Radiol 2017;18:132ŌĆō51.



19. Piscatelli N, Hyman N, Osler T. Localizing colorectal cancer by colonoscopy. Arch Surg 2005;140:932ŌĆō5.


20. Fritsch H, Brenner E, Lienemann A, Ludwikowski B. Anal sphincter complex: reinterpreted morphology and its clinical relevance. Dis Colon Rectum 2002;45:188ŌĆō94.


21. Papachrysostomou M, Pye SD, Wild SR, Smith AN. Anal endosonography in asymptomatic subjects. Scand J Gastroenterol 1993;28:551ŌĆō6.


22. Salerno G, Sinnatamby C, Branagan G, Daniels IR, Heald RJ, Moran BJ. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis 2006;8 Suppl 3:5ŌĆō9.


23. Sadahiro S, Ohmura T, Yamada Y, Saito T, Taki Y. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg Radiol Anat 1992;14:251ŌĆō7.










