- Search
| Ann Coloproctol > Volume 0(0); > Article |
Abstract
Purpose
This study aimed to identify risk factors for anastomotic leakage and to evaluate the impact of protective stoma on the rate of anastomotic leakage and subsequent management.
Methods
This retrospective study analyzed data from 4,282 patients who underwent low anterior resection between 2007 and 2014. Among these, 1,367 (31.9%) underwent surgery to create protective diverting stoma and 232 (5.4%) experienced anastomotic leakage. At 6-month timepoints, data were evaluated to identify any correlation between the presence of diverting stoma and the incidence of anastomotic leakage. In addition, clinicopathological parameters were investigated to identify risk factors for anastomotic leakage.
Results
Diverting stomas significantly reduced the rate of anastomotic leakage (hazard ratio, 0.334; 95% confidence interval, 0.212–0.525; P<0.001], which was reciprocally correlated with the rate of diverting stoma formation (P=0.039). Patients with a diverting stoma had a significantly lower incidence of generalized peritonitis (P<0.001) and therefore significantly reduced need for laparotomy (82.7% vs. 39.1%, P<0.001).
Anastomotic leakage is the most serious complication of low anterior resection for patients with rectal cancer; the rate of clinically significant complications has been reported to be as high as 20% [1]. Although many strategies have been used to reduce the rate of complications, the creation of defunctioning (diverting) stoma in this regard has been the subject of ongoing controversy. Some investigators have reported the protective effect of a stoma for low anterior resection in reducing the incidence of leakage and argue for its general use [2-4]. A previous meta-analysis of data from 4 randomized, controlled trials also showed a significant reduction in the number of clinically symptomatic leakages (odds ratio [OR], 0.32; 95% confidence interval [CI], 0.17–0.59) and fewer repeated operations (OR, 0.27; 95% CI, 0.14–0.51) in patients with a defunctioning stoma; the authors therefore recommended the use of stoma after low anterior resection in patients with rectal cancer [5]. By contrast, other investigators have suggested that the routine use of a diverting stoma is not advisable as their studies showed no reduction in the incidence of leakage [6]. There are many potential problems associated with the creation of stoma, including reduced quality of life, stoma-related morbidity [7], financial impact, secondary hospitalizations for stoma closure and the possibility of the patient requiring a permanent stoma [8]. Therefore, whether or not to use protective stoma as part of the surgical approach for low anterior resection of rectal lesions remains a key question. This study aimed to identify risk factors for anastomotic leakage and to evaluate the role of protective stoma on the rate of leakage and its management.
Between January, 2007, and December, 2014, 5,364 consecutive patients with rectal adenocarcinoma underwent surgery at the Asan Medical Center, Seoul, Korea. All patients had tumors located within 15 cm of the anal verge, as determined by colonoscopy and/or digital rectal examination. The clinicopathologic data for these patients were recorded in the hospital database and collected prospectively. We identified 4,282 patients who underwent low anterior resection; the remaining 1,082 patients were excluded for the following reasons: abdominoperineal resection (n=538), transanal local excision (n=260), bypass or diverting stoma formation only (n=109), Hartmann procedures (n=83), total colectomy or proctocolectomy (n=68), metachronous cancer (n=12), and concurrent inflammatory bowel diseases (n=12). Each patient provided informed consent prior to surgery.
All included patients received medical and mechanical bowel preparation, prophylactic subcutaneous heparin, and systemic antibiotic prophylaxis. Among the 4,282 patients, protective stomas were performed in 1,367 patients (31.9%); the decision to create a protective stoma was made by the attending surgeon.
The present study protocol was approved by the Institutional Review Committee of Asan Medical Center (2019-1366).
Patients were grouped according to the year in which they underwent surgery. We investigated the correlation between 2 values, the rate of anastomotic leakage and the rate of diversions performed during a period of time. Potential associations were identified by preparing a scatter plot with smooth lines of paired data.
To investigate possible risk factors for anastomotic leakage, 3 areas of clinicopathological data were reviewed: patient parameters (sex, age, and combined disease such as diabetes, hypertension, liver disease, pulmonary disease, cardiovascular disease, or chronic renal failure); disease parameters (location of tumors, level of carcinoembryonic antigen, pathologic T- and N-categories, tumor size, and the presence of lymphovascular invasion or perineural invasion); and treatment parameters (type of anastomosis [staple or hand-sewn], the application of minimally invasive techniques, diversion, and radiotherapy).
The tumor locations were categorized into 3 groups based on distance from the anal verge to the upper rectum (>10 cm, ≤15 cm), mid rectum (>5 cm, ≤10 cm), and lower rectum (≤5 cm). Three types of leakage were categorized as follows: generalized peritonitis, localized peritonitis with or without abscess around the anastomosis site, and any type of fistula or chronic sinus-associated leakage [9]. A long-term stoma was defined as one that could not be repaired for 1 year after its formation.
The clinicopathological characteristics of patients with or without any type of anastomotic leakage were compared using Pearson chi-squared test, Fisher exact test, or Student t-test depending on the nature of the data. Spearman Rank correlation coefficient was used to test the relationship between the diversion and anastomotic leakage. Cox regression analysis was used to determine the odds of anastomotic leakage using the significant parameters detected by univariate analysis.
The clinicopathological characteristics of the 4,282 enrolled patients, stratified by the year in which the low anterior resections were performed, are summarized in Table 1. The rate of anastomotic leakage and the rate of diversion were evaluated in 6-month groups. Between the 2 variables, Spearman Rank correlation coefficient was -0.520 and showed a statistically significant negative correlation (P=0.039). The rate of anastomotic leakage was found to reciprocally correlate with the number of diversions performed (Fig. 1).
Based on univariate analysis, 4 clinicopathological parameters (male sex, low level of tumor, radiotherapy, and diverting stoma) showed an association with the rate of anastomotic leakage (data not shown). In multivariate analysis using these 4 parameters, male sex (P<0.001), low level of tumor (mid rectum, P<0.001; low rectum, P<0.001)), and radiotherapy (P=0.01) were seen to be significant independent risk factors for anastomotic leakage. Additionally, a diverting stoma showed an independent effect in preventing anastomotic leakage (P<0.001) (Table 2). The protective role of diverting stoma for anastomotic leakage in low rectal cancer (≤5 cm from anal verge) patients who underwent radiotherapy (pre- or postoperatively) is summarized in Table 3. The diverting stoma for anastomotic leakage had protective effects in all cases, irrespective of receiving radiotherapy (male, 17.9% [22 of 123] vs. 5.7% [26 of 459]; female, 11.6% [10 of 86] vs. 5.1% [12 of 237]) or not (male, 13.8% [12 of 87] vs. 3.0% [3 of 100]; female, 10.7% [9 of 84] vs. 1.7% [1 of 60]).
The effects of diverting stoma on the type and management of anastomotic leakage, long-term stoma, and permanent stoma are summarized in Table 4. Patients with a diverting stoma had a significantly lower incidence of generalized peritonitis (P<0.001), and therefore significantly reduced requirement for laparotomy (82.7% vs. 39.1%, P<0.001). There was no statistically significant difference in the rate of long-term stoma between patients who underwent diverting stoma and those who did not (37.5% vs. 33.9%, P=0.165). The rate of permanent stoma did not differ significantly between the 2 groups, although a higher rate was seen in patients with a diverting stoma (46.9% vs. 34.5%, P=0.083).
Our study has shown that the creation of a diverting stoma significantly reduces the risk of anastomotic leakage and re-exploration rates in cases of anastomotic leakage. These results are similar to those seen in the literature [5, 10]. However, the methodology used in previous studies differs from our own; our study also included investigation of the correlation between the rate of diverting stoma performed and anastomotic leakage over time. Only the rate of diverting stoma procedures was seen to correlate with the rate of anastomotic leakage over time. By assessing the year in which procedures were performed at the same hospital, we could exclude the possibility of selection bias. Previous studies divided all patients into only 2 groups (stoma versus no stoma) and compared the rate of anastomotic leakage between these groups. This simple division of patients is likely to be a major cause of selection bias, due to significant differences between the cases included in each group. Furthermore, as the decision to construction a stoma is made at the surgeon’s discretion, it is difficult to draw strong conclusions as it is likely that more patients with risk factors for anastomotic leakage were included in the diverting stoma group, thereby forming the basis of selection bias. Therefore, if the rate of anastomotic leakage in the diverting group (in whom a risky anastomosis was anticipated) is similar to that seen in the nondiverting group, we could interpret these data as indicating the protective effect of diverting stoma.
In our study, the anastomotic leakage rate of the diverting group was not statistically significantly different from that of the nondiverting group (with diversion, 4.7% [64 of 1,367] vs. without diversion, 5.8% [168 of 2,915]). During the study periods, the rate of all types of leakage at our center was 5.4% (232 of 4,282). As shown in Table 1 and Fig. 1, the rate of anastomotic leakage was greatly affected by the rate of diversion procedures undertaken; the rate of anastomotic leakage could be increased if diverting stoma was performed in too few cases. We consider that it was not possible to avoid anastomotic leakage in all patients. Instead, specialist centers, or institutes for rectal cancer, should maintain the lowest possible rate of anastomotic leakage. Based on the results of our current analyses, it would be reasonable to construct a diverting stoma in approximately 30% of low anterior resections to maintain a rate of anastomotic leakage <5.0% at our center. In addition, these patients should include patients at high-risk of anastomotic leakage. A selective approach, using diverting stoma for high-risk patients to achieve the lowest possible leakage rates should, therefore, be considered.
Many factors have been reported to be risk factors for anastomotic leakage, including male sex, obesity, hypoalbuminemia, malnutrition, anemia, weight loss, a low-lying tumor requiring a low-level anastomosis, preoperative radiotherapy, adverse intraoperative events, and prolonged duration of surgery [11-14]. Here, we identified 3 significant independent risk factors: male gender, the use of radiotherapy, and a low tumor location, factors that are closely interrelated. As male gender is characterized by a narrow, concave bony pelvis (which limits access to a rectal tumor) and radiotherapy is often associated with low anastomosis, the low location of a rectal lesion could be the most important predictive factor for leakage. We therefore analyzed the role of diverting stoma in the subgroup of patients with low rectal cancer located within 5 cm of the anal verge. For low rectal cancers, the protective effects of a diverting stoma for anastomotic leakage was identified in all cases, irrespective of receiving radiotherapy or not. Although it is the subject of some controversy, preoperative radiotherapy is generally considered to be an independent risk factor for anastomotic leakage [15]. As preoperative radiotherapy is completed before surgical excision, this factor could be considered in the decision-making process. Postoperative radiotherapy, however, presents a more significant challenge. In our study, among the 95 low rectal cancer patients who received postoperative radiotherapy, 7 patients (7.4%) suffered from anastomotic leakage; 6 of the 55 patients (10.9%) without diverting stoma experienced leakages, while only 1 of the 40 patients (2.5%) with a diverting stoma experienced anastomotic leakage (Table 3). Therefore, an attending surgeon should consider the necessity of a diverting stoma when performing a low anterior resection in patients in whom postoperative radiotherapy is anticipated.
The treatment of anastomotic leakage after low anterior resection is challenging and some patients face the possibility of requiring a permanent stoma. In our study, anastomotic leakages were categorized into 3 types based on their characteristics. As the definitions are various and types of anastomotic leakage range from an asymptomatic leakage to a leakage resulting in a life-threatening condition, some authors have proposed different categorizations or grading systems. Rahbari et al. [16] proposed a 3-grade system according to its impact on clinical management (grade A, no change in patient management; grade B, requires active therapeutic intervention but is manageable without relaparotomy; and grade C, requires relaparotomy). Although this is a straightforward system, the grade could change after the completion of management for anastomotic leakage and thus cannot be used in the management process for complex clinical situations that vary over time. Chambers and Mortensen [9] proposed 3 categories of anastomotic leakage based on clinical presentation: a major leak with generalized peritonitis, a leak with localized peritonitis with or without abscess formation, and a fistula. As this system is based on clinical significance, it could support decision-making when developing an adequate management plan. This approach was adopted in our present study, with a modification that added chronic sinus as a fistula-type leakage.
For cases of anastomotic leakage that occurred without diverting stoma, 82.7% of patients required exploratory laparotomy, whereas only 39.1% of patients in the diverting group required laparotomy (Table 4). In the group without diverting stoma, all patients with generalized peritonitis received laparotomy to manage generalized peritonitis. However, in the diverting group, laparotomy was mainly performed for the re-exploration of localized peritonitis (n=10) and fistulas (n=10) that occurred after closing the diverting stoma. Although recent improvements in perioperative management have been made, generalized peritonitis with sepsis that requires exploration remains a major cause of mortality. The adequate use of diverting stoma could significantly reduce the rates of these septic complications.
One of the main sequelae associated with anastomotic leakage is the possibility of a permanent stoma. In our study, the stomas of 81 cases (1.9% of the total cohort of 4,282 patients, and 34.9% of the 232 patients with leakage) could not be repaired after 1 year; most of these long-term stomas became permanent. There was no statistically significant difference in the rate of long-term stoma between the diverting group and the nondiverting group (37.5% vs. 33.9%, P=0.165). However, the rate of permanent stoma was higher in the diverting group than nondiverting group (46.9% vs. 34.5%, P=0.083). As cases of rediversion in the diverting group usually underwent management for anastomotic leakage that occurred after repair of the primary stoma, the decision to repair the primary stoma and to perform rediversion should be considered carefully.
In conclusion, anastomotic leakage is a serious postoperative complication that can occur after low anterior resection for rectal cancer. Our data suggest that the selective use of diverting stoma in high-risk patients could reduce the rate of anastomotic leakage. The risk factors for anastomotic leakage were male sex, radiation therapy, and a low rectal cancer location. Diverting stomas showed protective effects in these high-risk patients, also reducing the need for emergency laparotomy by approximately 40%.
Fig. 1.
The reciprocal correlation between the rates of diversion performed and anastomotic leakage.
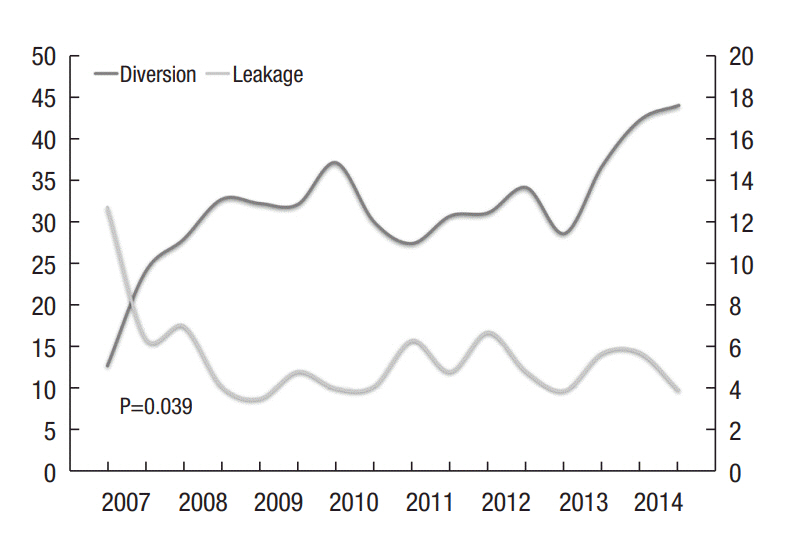
Table 1.
Clinicopathologic characteristics of patients according to years
Table 2.
Multivariate analysis for risk factors of anastomotic leakage
Table 3.
The protective role of diverting stoma for anastomotic leakage in low rectal cancer (≤5 cm from anal verge) underwent low anterior resection and radiotherapy
Table 4.
Effect of diverting stoma on type and management of anastomotic leakage and long-term stoma
| Variable | Diversion (−) (n = 2,915) | Diversion (+) (n = 1,367) | P-value |
|---|---|---|---|
| No. of leakages | 168 (5.8) | 64 (4.7) | 0.145 |
| Type of leakage | |||
| Generalized peritonitis | 84 (50.0) | 3 (4.7) | < 0.001 |
| Localized peritonitis (abscess) | 56 (33.3) | 36 (56.3) | |
| Fistula or chronic sinus | 28 (16.7) | 25 (39.1) | |
| Management of leakage | |||
| Explo-laparotomy | 139 (82.7) | 25 (39.1) | < 0.001 |
| Conservative management | 25 (14.9) | 29 (45.3) | |
| PCD | 4 (2.4) | 10 (15.6) | |
| Long-term stomaa | 57 (33.9) | 24 (37.5) | 0.610 |
| Permanent stoma | 58 (34.5) | 30 (46.9) | 0.083 |
REFERENCES
1. Harris LJ, Phillips BR, Maxwell PJ, Isenberg GA, Goldstein SD. Outcomes of low anterior resection anastomotic leak after preoperative chemoradiation therapy for rectal cancer. Am Surg 2010;76:747–51.


2. Eriksen MT, Wibe A, Norstein J, Haffner J, Wiig JN; Norwegian Rectal Cancer Group. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis 2005;7:51–7.


3. Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 2005;92:211–6.


4. Cong ZJ, Hu LH, Zhong M, Chen L. Diverting stoma with anterior resection for rectal cancer: does it reduce overall anastomotic leakage and leaks requiring laparotomy? Int J Clin Exp Med 2015;8:13045–55.


5. Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008;248:52–60.


6. Enker WE, Merchant N, Cohen AM, Lanouette NM, Swallow C, Guillem J, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg 1999;230:544–52.



7. Rullier E, Le Toux N, Laurent C, Garrelon JL, Parneix M, Saric J. Loop ileostomy versus loop colostomy for defunctioning low anastomoses during rectal cancer surgery. World J Surg 2001;25:274–7.


8. Bailey CM, Wheeler JM, Birks M, Farouk R. The incidence and causes of permanent stoma after anterior resection. Colorectal Dis 2003;5:331–4.


9. Chambers WM, Mortensen NJ. Postoperative leakage and abscess formation after colorectal surgery. Best Pract Res Clin Gastroenterol 2004;18:865–80.


10. Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 2009;96:462–72.


11. Iancu C, Mocan LC, Todea-Iancu D, Mocan T, Acalovschi I, Ionescu D, et al. Host-related predictive factors for anastomotic leakage following large bowel resections for colorectal cancer. J Gastrointestin Liver Dis 2008;17:299–303.

12. Jestin P, Påhlman L, Gunnarsson U. Risk factors for anastomotic leakage after rectal cancer surgery: a case-control study. Colorectal Dis 2008;10:715–21.


13. Mäkelä JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum 2003;46:653–60.


14. Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998;85:355–8.


- TOOLS
-
METRICS

- Related articles in ACP
-
Quality of Life After a Low Anterior Resection in Elderly Patients2016 February;32(1)
The Role of Diverting Stoma After an Ultra-low Anterior Resection for Rectal Cancer2013 April;29(2)
A Study of Anal Manometric Finding after Low Anterior Resection of Rectal Cancer.2000 October;16(5)







