- Search
| Ann Coloproctol > Volume 37(5); 2021 > Article |
|
Abstract
Methods
All consecutive patients who had consulted between May 1, 2016 and June 30, 2017 for bleeding hemorrhoidal disease were prospectively assessed at a proctological department. The study was conducted in 2 stages. The first stage assessed the validity of the score on a prospective patient cohort. A second stage assessed the interobserver reproducibility of the score on another prospective cohort.
Results
One hundred consecutive patients were studied (57 males; mean age, 49.70 years). A positive association between HBS and surgery indication was found (P<0.001). A cut-off value of the score of 5 (≤5 vs. >5) separated patients from surgical to medical-instrumental treatment with a sensitivity and specificity of 75.00% and 81.25%, respectively. In the multivariate analysis, only HBS was significantly associated with the operative decision (odds ratio, 12.22). Prolapse was no longer significantly associated with the surgical indication. After a mean follow-up after treatment of 7 months, HBS improved statistically significantly (P<0.0001). For the reproducibility of the score, an additional 30 consecutive patients (13 males; mean age, 53.14 years) were enrolled with an excellent agreement between 2 proctologists (kappa=0.983).
Conclusion
HBS is sensitive, specific, and reproducible. It can assess the severity of hemorrhoidal bleeding. It can discriminate between the most severe surgery-indicated patients and does so in a more efficient way than the Goligher prolapse score. It also allows quantifying the extent of change in hemorrhoidal bleeding after treatment.
Hemorrhoidal disease is a frequent disease whose usual symptoms are thrombosis, prolapse, and/or bleeding. These symptoms are one of the most common reasons for consultation, especially as they are a source of great anxiety for patients and therefore of deterioration in quality of life. Bleeding is often recurrent and/or abundant, sometimes resulting in iron deficiency or even anemia. As a result, therapeutic management of hemorrhoidal disease is highly variable, ranging from simple medical treatment to conventional or minimally-invasive surgery, to instrumental treatment, and very recently in selected cases, to radiological embolization of the superior rectal arteries [1].
The degree of hemorrhoid prolapse can be quantified by the 1984 Goligher prolapse score (1 to 4), which is simple and reproducible [2]. However, to our knowledge, there is no validated score making it possible to specifically characterize bleeding. Such a score could be potentially useful to the nonspecialist physician in proposing the most appropriate treatment to his/her patient. It would also make it possible to assess the efficacy of various treatments for hemorrhoidal disease, some of which are sometimes very effective on bleeding. Our multidisciplinary team, while developing many new minimally-invasive hemorrhoidal surgery techniques (Doppler-guided artery ligation with mucopexy, radiofrequency, and laser hemorrhoidoplasty) and hemorrhoid embolization, was faced with the absence of an objective assessment tool of hemorrhoidal bleeding in the literature.
We therefore designed a hemorrhoidal bleeding score (HBS) (Table 1) and applied it initially to the evaluation of the embolization results [3, 4].
However, before this score can be used more widely in comparative studies of hemorrhoidal bleeding treatment techniques, it needs to be validated. The aim of this prospective study was to evaluate whether this score was able to predict the need for surgery, sensible to bleeding change, and reproducible.
All consecutive patients, aged 18 years or over, who had consulted for hemorrhoidal disease responsible for isolated bleeding or associated with other symptoms of hemorrhoidal disease, were prospectively assessed in our tertiary center dedicated to medical-instrumental and surgical proctology. According to the French guidelines [5], a colonic exploration was always performed after the age of 45 years and in the case of diagnostic uncertainty.
The non-inclusion criteria were other associated anal diseases as fissure or fistula, the absence of social security coverage, inability to understand French, refusal of follow-up, pregnancy, need for an antiaggregant or an anticoagulant treatment, known hemostasis or coagulation disorders, anal incontinence, and uncontrolled chronic inflammatory bowel disease.
The items of this score were chosen by the coauthors [3] because they were considered to be the most relevant.
Anemia was defined as hemoglobin less than 13 g/dL in males and less than 12 g/dL in females. Iron deficiency was defined as a serum ferritin level below 30 ng/mL. The need for a blood transfusion was left to the discretion of the doctors who took care of the patient according to the usual criteria (hemodynamic tolerance, age, coronary history, vascular history, anticoagulant treatments, etc.). The degree of discomfort associated with hemorrhoidal disease was rated by the patient on a visual analogue scale (VAS) ranging from 0 to 10: VAS score 0 to 3, little or no discomfort; 4 to 6, moderate discomfort; and 7 to 10, frank or permanent discomfort.
The study was conducted in 2 stages. The first stage aims at assessing the validity of the score on a prospective patient cohort. The second stage was designed to assess the interobserver reproducibility of the score on another prospective patient cohort.
Patients were seen in a scheduled consultation at the Paris SaintJoseph Hospital by the proctologist surgeon designated as the study reference person (NF) and were included if they met the study inclusion/noninclusion criteria. A pretreatment data collection sheet, including the HBS and the Goligher prolapse score, was completed by the proctologist surgeon (NF) in consultation for each patient at the time of inclusion. A surgical or medical-instrumental treatment was proposed according to the latest French recommendations for the treatment of hemorrhoidal disease [5]. Surgery was proposed to all patients with a Goligher score of 4. In the case of a Goligher score of 3, the surgical indication depended on the circumference of the prolapse. For example, in the case of a prolapse score of 3 of a single hemorrhoidal pile, rubber band ligation was proposed. Surgical treatment consisted of either a single- or a multi-pile hemorrhoidectomy or a Doppler-guided arterial ligation with mucopexy. Medical treatment consisted of topical agents, venotonic agents, and/or stool softeners. Instrumental treatment consisted of infrared coagulation and/or rubber band ligation.
For the first cohort of patients aiming at assessing the validity of the score, the data collection sheet was completed by the same proctologist surgeon (NF) at the posttreatment follow-up consultation, thus enabling the pre- and posttreatment HBS to be obtained. Patients not seen in consultation were interviewed by telephone.
For the second cohort aiming at assessing the interobserver reproducibility of the score, there was no collection of proposed treatments or post-therapeutic follow-up. After questioning the patient, the referent proctologist surgeon (NF) completed the pretreatment data collection sheet, including the HBS, in the presence of another proctologist (JM). The latter did not participate in the interrogation of the patient but completed another data collection sheet including the HBS, based on the patient’s responses to NF and without knowledge of NF’s notes. This same-day simultaneous assessment was chosen because hemorrhoidal symptoms vary from day to day.
The accuracy of the HBS was evaluated by using the decision of surgery as the gold standard. Sensitivity was defined as its ability to detect the presence of a surgical indication, and its specificity as its ability to detect the absence of surgical indication. The distribution of indications for each score value were also recorded. The Youden index [6], measuring the accuracy of the score to predict surgery, was calculated using sensitivity and specificity values as follows: ‘sensitivity + specificity – 1’. The highest Youden index value is the point that best distinguishes patients from nonpatients. In the present study, we defined ‘patients’ as subjects who had undergone surgery and ‘nonpatients’ as those who had not. The Mann-Whitney test was used to test association of surgery with both the HBS and the Goligher prolapse score.
The comparison of the Goligher prolapse score and the HBS was assessed visually by comparing the 2 receiver operating characteristic (ROC) curves [7] and statistically by the associated test, using the DeLong method [8].
For multivariate analysis, a logistic model was developed using the decision of surgery as the dependent variable and age, sex, Goligher prolapse score, and HBS. For this analysis, the HBS was binarized (1 to 5 vs. 6 to 9) as well as the Goligher prolapse score (grade 1 and 2 vs. grade 3 and 4). Interobserver agreement was assessed using a weighted kappa coefficient. All analyses were performed using R software, ver. 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). A P-value of 0.05 was chosen as the significance level.
The study sponsor of this investigator-initiated study was the Paris Saint-Joseph Hospital. The study was approved by our Institutional Ethics Committee (No. 1072 and initial agreement No. 2016-03-06). The ClinicalTrials.gov identifier is NCT03060616.
According to the French Law (Loi Jardé), all patients were given an information sheet and declared their non-opposition which was recorded in their medical record. The authors vouch for the completeness and accuracy of the data and analyses and for the fidelity of the trial to the protocol.
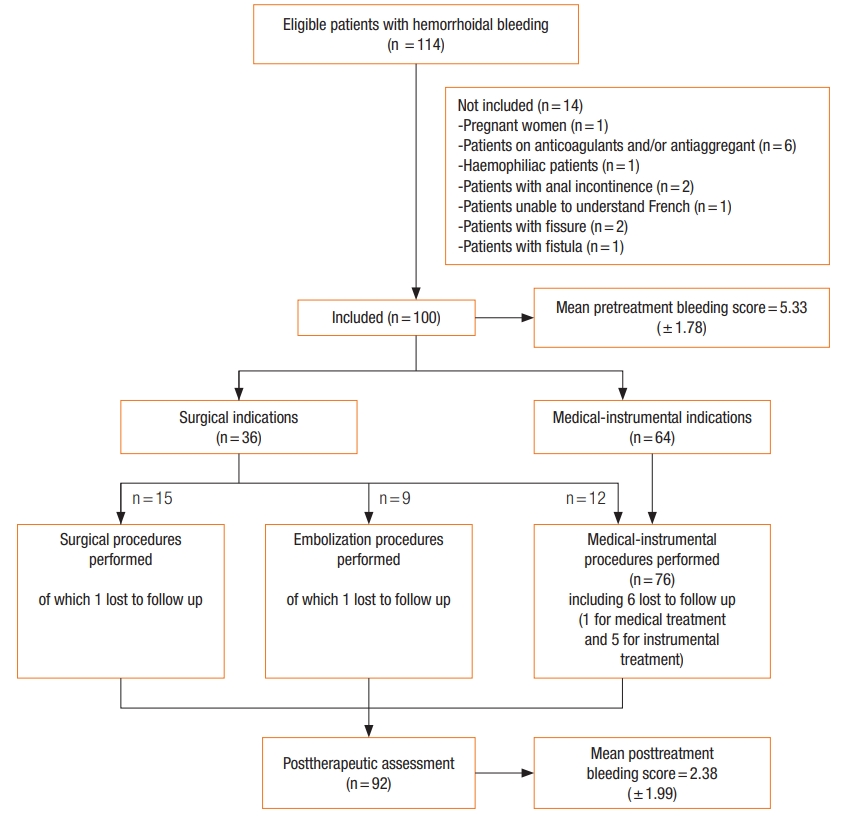
Between May 1, 2016 and June 30, 2017, 114 consecutive patients consulted for hemorrhoidal bleeding. Fourteen were not included in the study (Fig. 1). The age (mean± standard deviation) of the 100 patients (57 males) was 49.70± 13.70 years. Four patients had a history of hemorrhoidal surgery. Hemorrhoidal bleeding was associated with prolapse (63.0%), seepage soiling the underwear (29.0%), bothersome skin tags (17.0%), and external thrombosis (6.0%). Prior to treatment, the HBS was 5.33± 1.78) and the Goligher prolapse score was equal to 1, 2, 3, and 4 among 32, 5, 57, and 6 patients, respectively.
Surgical treatment was decided for 36 patients. There was a strong relationship between the HBS value and indication for surgical treatment: 7 (interquartile range [IQR], 5.5 to 8) vs. 4 (IQR, 4 to 5); P< 0.001 (Fig. 2). There was no indication for surgical treatment when HBS was ≤ 3. There was a surgical indication in 25.4% (16 of 63) of the patients with a score of between 4 and 6 and in 76.9% (20 of 26) of the patients with a score of ≥ 7 (Fig. 2).
Sensitivity, specificity, and Youden index for each HBS value are presented in Table 2. The Youden index and the ROC curve (Fig. 3) made it possible to retain the HBS value of 5 (≤ 5 vs. > 5) as the best threshold for separating patients with an indication for surgical treatment from those with an indication for medical-instrumental treatment. At this threshold, sensitivity and specificity were 27 of 36 (75.0%; 95% confidence interval [CI], 0.59 to 0.86) and 52 of 64 (81.3%; 95% CI, 70.00 to 88.90), respectively. Hence, surgery was proposed in 14.8% (9 of 61) of patients with a score of ≤ 5 vs. 69.2% (27 of 39) of patients with a score of > 5 (chi-square= 30.60, degree of freedom= 1; P< 0.0001).
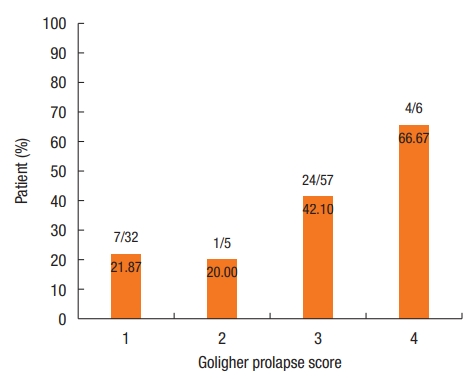
Fig. 4 shows the number (percentage) of patients for whom there was an indication for surgical treatment according to Goligher prolapse score.
In the multivariate analysis, only the HBS was significantly associated with the indication for surgical treatment (odds ratio, 12.22; 95% CI, 3.23 to 46.27). Prolapse was no longer significantly associated with the indication for surgical treatment (Table 3).
Medical treatment was given to 9 patients and instrumental treatment to 55 patients. All of these patients agreed to the proposed therapy. Of the 36 patients for whom the surgical indication was given, 15 accepted the proposed therapy; either medical or instrumental treatment was initiated or hemorrhoidal embolization was performed in those who refused surgical treatment (Fig. 1).
The follow-up after treatment was 7± 4 months. Of the 100 patients included, 8 patients were lost to follow-up (after medical treatment, 1; instrumental treatment, 5; embolization, 1; or single pile hemorrhoidectomy, 1). Among the remaining 92 follow-up patients, 39 were seen in consultation and 43 were contacted by telephone. A statistically significant improvement in HBS and Goligher prolapse score was observed after treatment (Table 4).
From October 1 to 31, 2017, an additional 30 consecutive patients (13 males) with age of 53.14± 13.82 years were enrolled. Their HBS was 5.36± 2.07 for the first proctologist (NF) vs. 5.30± 2.11 for the second proctologist (JM), with an excellent agreement (weighted kappa= 0.983). The score was the same for all but 4 patients. For these patients, the difference did not exceed one point: score of 4 for JM and 3 for NF (n= 1), score of 2 for JM and 3 for NF (n= 1), and score of 4 for JM and 3 for NF (n= 2).
Our study made it possible to propose a specific HBS with good diagnostic properties and excellent interobserver reproducibility. To our knowledge, this HBS is the first of its kind.
The calculation of this score is based on simple clinical items and objective biological criteria. It also considers the overall perception of the patient, which enables his/her degree of satisfaction with the proposed treatment to be measured. We evaluated it in 100 consecutive patients who consulted our specialized tertiary center for the treatment of proctological diseases.
Our study population differs from the previous 2 studies that have already used this HBS [3, 4]. In fact, in these 2 studies, patients on antiaggregants and/or anticoagulants or with known hemostasis and/or coagulation disorders were included (16% in the study by Tradi et al. [4] and 68% in the study by Moussa et al. [3]). In the study by Moussa et al. [3], the patients included were the most severe (mean pretreatment HBS was 7 [6-8]) and most of them had multiple contraindications to surgery. In our study, these patients were not included so as to have a study population closer to that encountered in daily practice, thereby reducing the tertiary center effect.
Numerous evaluation scores for hemorrhoidal disease are reported in the literature. Some authors sought to extend Goligher prolapse score to include PATE (prolapse, acute symptoms, anal tone, external pile) scores [9, 10] or the single pile classification score [11]. Other authors proposed composite scores that are most often unvalidated and not consensual [12], taking into account several components of hemorrhoidal disease. This is the case for the Symptom Score [13], the classification of hemorrhoids as proposed by Lunniss and Mann [14], the Symptom Questionnaire by Thaha et al. [15], the hemorrhoid severity score (HSS) [16], the symptom questionnaire by Giordano et al. [17], and the Sodergren hemorrhoid symptom severity score [18] which moreover does not take into account hemorrhoidal bleeding. Finally, scores also assessed patients’ quality of life, such as the Hemorrhoidal Disease Symptom Score, a new version of the HSS score [19] or the HEMO-FISS-QoL (Hemorrhoid and Fissure Quality of Life) score [20]. However, these various scores do not have widespread use, probably because they are excessively complex for routine practice. Above all, no score specifically focused on the characterization of hemorrhoidal bleeding, which is the most common cause of consultation for hemorrhoidal disease.
The HBS we have developed has an immediate clinical impact. It can be used to measure objectively the severity of hemorrhoidal bleeding. Indeed, these bleeds are often difficult to assess with precision, particularly by general practitioners or gastroenterologists/surgeons not familiar with proctological diseases, especially as patients tend to overestimate their bleeding. Given the frequency of hemorrhoidal disease, the use of such a simple-to-administer score, including by telephone, could serve as a tool for objective exchange between the different healthcare professionals involved in the management of these patients.
In addition, for a nonspecialist practitioner, this score can also be an aid for the optimal therapeutic choice to propose (medicalinstrumental vs. surgical). Furthermore, the Goligher prolapse score was criticized for its weak association with other hemorrhoidal symptoms and quality of life [21] and our study showed that the degree of prolapse seemed to weigh less in the surgical indication than the HBS. It is likely that abundant bleeding with anemia causes more concern to the patient and practitioner than a Goligher grade III or IV prolapse. Taking into account the results of our study, the patients could be referred to a proctological surgeon from a HBS of greater than 5, while medical-instrumental treatment would be sufficient for a score of less than or equal to 5. Finally, this HBS makes it possible to evaluate the efficacy of the various treatments available for hemorrhoidal disease, in particular those which act primarily on bleeding. We have already demonstrated this for hemorrhoidal arterial embolization [3, 4].
Our study has some limitations. It was conducted in a tertiary center, possibly with patients with more severe pathology and surgical indications than usual. A limited number of patients were assessed (n= 100), especially as 8 were lost to follow-up, and with a short follow-up. The assessment was done by a single proctologist responsible for the therapeutic indications. Even if the choice was based on the French recommendations for the treatment of hemorrhoidal disease [5], the latter are built only on the Goligher score and do not take into consideration; for example, the quality of life of patients. Besides, 21 out of 36 patients (58.3%) refused the proposed surgery because they fear the postoperative consequences. Moreover, among the 92 patients followed up, 39 patients only underwent clinical examination while 43 were phone called. Despite the sensitivity of the HBS to identify patients with a surgical indication, it was insufficient on its own to distinguish patients requiring only medical treatment from those requiring instrumental treatment. This HBS must therefore be improved, in particular by the different weighting of some of its items, in order to help us choose between the different approaches of hemorrhoidal disease. It would also deserve to be used within a consensual composite score to assess hemorrhoidal disease overall, and therefore also including a prolapse subscore (Goligher for example), an external hemorrhoidal disease subscore (currently nonexistent), a specific quality-of-life subscore, and patient-reported outcomes [1, 22].
The HBS is sensitive, specific, and reproducible. It can be used to assess the severity of hemorrhoidal bleeding. It can discriminate between the most severe surgery-indicated patients and does so in a more efficient way than Goligher prolapse score. Finally, it can quantify the decrease in hemorrhoidal bleeding under treatment. Now, we have to include it in a composite score to assess hemorrhoidal disease in a more general and consensual way.
ACKNOWLEDGMENTS
The study sponsor of this investigator-initiated study was the Paris Saint-Joseph Hospital.
Fig. 2.
Percentage of surgery-indicated patients according to the hemorrhoidal bleeding score (HBS) value.

Fig. 3.
Receiver operating characteristic (ROC) curve; sensitivity and specificity of the hemorrhoidal bleeding score.
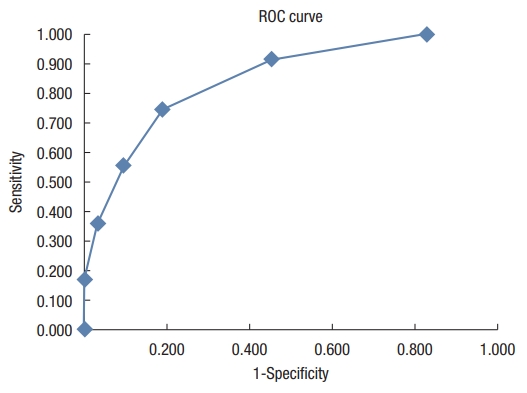
Table 1.
Hemorrhoidal bleeding score
Table 2.
Specificity, sensitivity, and Youden index of the hemorrhoidal bleeding score (HBS) among 100 patients
Table 3.
Multivariate logistic regression analysis of the need for surgical treatment (n=100)
| Variable | Coefficient | SE | P-value | Odds ratio (95% CI) |
|---|---|---|---|---|
| Constant | –2.97 | 1.25 | 0.02* | - |
| Age (yr) | –0.004 | 0.02 | 0.82 | 1.00 (0.96–1.04) |
| Male sex | 0.51 | 0.51 | 0.32 | 1.66 (0.61–4.52) |
| Goligher prolapse score (grade 1 and 2) | 0.73 | 0.55 | 0.18 | 2.08 (0.71–6.15) |
| HBS (1–5) | 2.50 | 0.68 | < 0.01* | 12.22 (3.23–46.27) |
Table 4.
Change in pre- and posttreatment bleeding and Goligher prolapse scores among 100 patients
| Variable | Pretreatment | Posttreatment | Difference (95% CI) | t-value | P-value |
|---|---|---|---|---|---|
| Hemorrhoidal bleeding score | 5.33 ± 1.78 | 2.38 ± 1.99 | 2.95 (2.49–3.45) | 12.25 | < 0.0001* |
| Goligher prolapse score | 2.36 ± 1.01 | 2.07 ± 1.02 | 0.29 (0.11–0.46) | –3.22 | 0.002* |
REFERENCES
1. Sandler RS, Peery AF. Rethinking what we know about hemorrhoids. Clin Gastroenterol Hepatol 2019;17:8–15.


2. Goligher JC. Haemorrhoids or piles. In: Goligher JC, editor. Surgery of the anus, rectum and colon. 4th ed. London: Bailliere Tindall; 1980. p. 96.
3. Moussa N, Sielezneff I, Sapoval M, Tradi F, Del Giudice C, Fathallah N, et al. Embolization of the superior rectal arteries for chronic bleeding due to haemorrhoidal disease. Colorectal Dis 2017;19:194–9.


4. Tradi F, Louis G, Giorgi R, Mege D, Bartoli JM, Sielezneff I, et al. Embolization of the superior rectal arteries for hemorrhoidal disease: prospective results in 25 patients. J Vasc Interv Radiol 2018;29:884–92.


5. Higuero T, Abramowitz L, Castinel A, Fathallah N, Hemery P, Laclotte Duhoux C, et al. Guidelines for the treatment of hemorrhoids (short report). J Visc Surg 2016;153:213–8.


6. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005;47:458–72.


7. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77.



8. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45.


9. Gaj F, Trecca A. PATE 2000 Sorrento: a modern, effective instrument for defining haemorrhoids. A multicentre observational study conducted in 930 symptomatic patients. Chir Ital 2004;56:509–15.

10. Gaj F, Trecca A. New “PATE 2006” system for classifying hemorrhoidal disease: advantages resulting from revision of “PATE 2000 Sorrento”. Chir Ital 2007;59:521–6.

11. Elbetti C, Giani I, Novelli E, Fucini C, Martellucci J. The single pile classification: a new tool for the classification of haemorrhoidal disease and the comparison of treatment results. Updates Surg 2015;67:421–6.


12. Rubbini M, Ascanelli S. Classification and guidelines of hemorrhoidal disease: present and future. World J Gastrointest Surg 2019;11:117–21.



13. Shanmugam V, Thaha MA, Rabindranath KS, Campbell KL, Steele RJ, Loudon MA. Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids. Cochrane Database Syst Rev 2005;(3): CD005034.



14. Lunniss PJ, Mann CV. Classification of internal haemorrhoids: a discussion paper. Colorectal Dis 2004;6:226–32.


15. Thaha MA, Campbell KL, Kazmi SA, Irvine LA, Khalil A, Binnie NR, et al. Prospective randomised multi-centre trial comparing the clinical efficacy, safety and patient acceptability of circular stapled anopexy with closed diathermy haemorrhoidectomy. Gut 2009;58:668–78.


16. Nyström PO, Qvist N, Raahave D, Lindsey I, Mortensen N; Stapled or Open Pile Procedure (STOPP) trial study group. Randomized clinical trial of symptom control after stapled anopexy or diathermy excision for haemorrhoid prolapse. Br J Surg 2010;97:167–76.


17. Giordano P, Nastro P, Davies A, Gravante G. Prospective evaluation of stapled haemorrhoidopexy versus transanal haemorrhoidal dearterialisation for stage II and III haemorrhoids: three-year outcomes. Tech Coloproctol 2011;15:67–73.



18. Pucher PH, Qurashi M, Howell AM, Faiz O, Ziprin P, Darzi A, et al. Development and validation of a symptom-based severity score for haemorrhoidal disease: the Sodergren score. Colorectal Dis 2015;17:612–8.


19. Rørvik HD, Styr K, Ilum L, McKinstry GL, Dragesund T, Campos AH, et al. Hemorrhoidal Disease Symptom Score and Short Health ScaleHD: new tools to evaluate symptoms and health-related quality of life in hemorrhoidal disease. Dis Colon Rectum 2019;62:333–42.


20. Abramowitz L, Bouchard D, Siproudhis L, Trompette M, Pillant H, Bord C, et al. Psychometric properties of a questionnaire (HEMO-FISS-QoL) to evaluate the burden associated with haemorrhoidal disease and anal fissures. Colorectal Dis 2019;21:48–58.


- TOOLS