- Search
|
|
Abstract
Purpose
Malignant large bowel obstruction is a surgical emergency that requires urgent decompression. Stents are increasingly being used, though reported outcomes are variable. We describe our multidisciplinary experience in using stents to manage malignant large bowel obstruction.
Methods
All patients undergoing colorectal stent insertion for acute large bowel obstruction in a teaching hospital were included. Outcomes, complications, and length of stay (LOS) were recorded.
Results
Over a 7-year period, 73 procedures were performed on 67 patients (37 male, mean age of 76 years). Interventional radiology was involved in all cases. Endoscopic guidance was required in 24 cases (32.9%). In 18 patients (26.9%), treatment intent was to bridge to elective surgery; 16 had successful stent placement; all had subsequent curative resection (laparoscopic resection, 8 of 18; primary anastomosis, 14 of 18). Overall LOS, including both index admission and elective admission, was 16.4 days. Treatment intent was palliative in 49 patients (73.1%). In this group, stents were successfully placed in 41 of 49 (83.7%). Complication rate within 30 days was 20%, including perforation (2 patients), per rectal bleeding (2), stent migration (1), and stent passage (5). Nineteen patients (38.8%) required subsequent stoma formation (6, during same admission; 13, during subsequent admission). Overall LOS was 16.9 days.
Conclusion
In our experience colorectal stents can be used effectively to manage malignant large bowel obstruction, with only selective endoscopic input. As a bridge to surgery, most patients can avoid emergency surgery and have a primary anastomosis. In the palliative setting, the complication rate is acceptable and two-thirds avoid a permanent stoma.
Patients with colorectal cancer may present acutely with large bowel obstruction (LBO) in over 10% of cases [1] and this can be a challenging condition to manage. A range of treatment options are available. Treatment intent may be curative or palliative; decompression of the bowel may be achieved surgically or by placement of a stent through endoscopic, radiological, or combined approaches. Stent placement may avoid a stoma both as a bridge to curative surgery [2] and in the palliative setting [3]. Though less invasive and with an initial technical success rate of up to 94% reported [4], clinical failure has been reported in over 50% including migration, obstruction, perforation and tenesmus [4, 5], and a reduction in morbidity and mortality compared to surgery has not been demonstrated [2, 5]. The paucity of high quality evidence makes this an important area for ongoing research. We describe our single-center experience with colorectal stenting in malignant LBO.
In this retrospective study, inclusion criteria were patients undergoing colorectal stent insertion for acute malignant LBO between February 2010 and April 2017. Exclusion criteria were stents inserted for a benign indication. Malignant bowel obstruction was diagnosed by computed tomography (CT) scan with the following diagnostic criteria: distension of the proximal bowel, an obstructing lesion at the transition point, and relatively collapsed bowel downstream. Informed consent was obtained from all patients for the procedure being performed. This study was an evaluation of service and therefore an approval of the institutional review board was not required. Research analysis of the data was prospectively approved by Northern Ireland Research Ethics Committee (No. 18/NI/0138). Data collection included method of stent insertion (radiological and/or endoscopic), duration of procedure, technical success rate (defined as successful stent insertion), clinical success rate (defined as the resumption of bowel function and the avoidance of emergency surgery), stent migration (defined as movement of the stent from the initial site of insertion), stent passage (defined as the stent being passed per rectum [PR]), complications, length of stay (LOS), stoma rate, and need for subsequent surgery. Patients were analyzed in 2 groups according to intention of treatment: (1) curative, with the intention to bridge to curative elective surgery; and (2) palliative, with the intention to palliate the symptoms of bowel obstruction. Statistical analysis was performed using Microsoft Excel (Microsoft, Redmond, WA, USA).
Seventy-three procedures were performed on 67 patients (37 male, mean age of 76 years), including 4 patients who had 2 stents inserted and 1 patient who had three stents inserted over the course of the study period. Distribution of tumor location was as follows: rectum, 5 patients; rectosigmoid, 15; sigmoid colon, 30; descending colon, 10; splenic flexure, 1; and transverse colon, 6.
Mean time from request to procedure was 26 hours and procedure duration was 82 minutes. Interventional radiology was involved in all cases, whereby a guide-wire was passed across the malignant stricture under radiological guidance and a stent passed over the guide-wire. Endoscopic support, by advancing the endoscope to the stricture and passing the guide-wire under endoscopic vision, was used in 24 procedures (32.9%). All transverse colon and descending colon stents were placed with endoscopic guidance (Fig. 1), with the exception of one transverse colon stent, which was placed via an end colostomy in a patient who had had a previous Hartmann procedure. Overall technical success rate, defined as satisfactory stent placement, was 85.1% (57 of 67). Of those who had stents successfully inserted, clinical success rate, defined as the resumption of bowel function and the avoidance of emergency surgery during the index admission, was 83.1% (49 of 59). In 72.6% of procecures (53 of 73), an abdominal radiograph was performed postprocedure (mean time, 17 hours), showing a satisfactory position in 98.6% (1 stent had been expelled) and radiological improvement of obstruction in 78%.
In 18 patients (26.9%), treatment intent was to bridge to elective surgery (Table 1). Sixteen patients (88.9%) had successful stent placement, one of whom re-obstructed. All patients had subsequent curative resection, performed electively in 15 of 18 (83.3%), laparoscopically in 8 of 18 (44.4%) and with primary anastomosis in 14 of 18 (77.8%). A primary anastomosis was not performed in 4 patients: 2 patients were octogenarians and decided against an anastomosis, 1 patient was found to have a perforated rectal tumor intraoperatively and an anastomosis was not done, and 1 patient underwent an emergency Hartmann procedure following reobstruction. Three patients had open low anterior resection and loop ileostomy formation, which were all subsequently closed. Long-term stoma rate was 4 of 18 (22.2%). Overall LOS was 16.4 days.
Treatment intent was palliative in 49 patients (73.1%), and this decision was based on the presence of irresectable metastatic disease in 31 patients (63.3%) and significant medical comorbidity in the others. In this group, stents were successfully placed in 41 of 49 (83.7%). Of the 8 patients in whom stent placement was unsuccessful, 4 had stoma formation during the same admission and 4 were managed conservatively. Complication rate within 30 days was 20%, including perforation (2 patients, 4.1%), PR bleeding (2 patients, 4.1%), stent migration (1 patient, 2.0%), and stent passage PR (5 patients, 10.2%). Of those with stent-related perforation, 1 patient died and 1 patient went on to have a Hartmann procedure. Overall 19 of 49 patients (38.8%) required stoma formation: 6, during same admission; 13, during subsequent admission. Median time to readmission and stoma formation in these patients was 9 months.
Compared to patients in the curative group, patients in the palliative group were more likely to have comorbidities and metastatic disease. Complication rate was similar. During the study period, the same physicians were involved in patient care and there was no change in stent-related complications over that period.
Of the 5 patients with rectal cancers, 2 were in the mid-rectum and 3 were in the upper rectum. All patients tolerated the stent well. One patient was in the curative group and later underwent anterior resection and primary anastomosis. Of the 4 patients in the palliative group, 1 patient had a PR bleed.
To determine whether the use of endoscopic guidance affected clinical outcomes, patient characteristics and outcomes were compared (Table 2). Procedure duration was significantly longer with endoscopic guidance; there were no differences in patient’s age and sex, technical failure, or need for emergency surgery.
Malignant LBO can be challenging to manage. The patient group is heterogeneous, with some patients requiring curative treatment and others palliative, and with a range of treatment modalities available. Colorectal stent insertion forms part of the National Institute for Health and Care Excellence (NICE) guideline for managing colorectal cancer [6]. Though a body of evidence is accumulating, reported outcomes and complications vary greatly, and the role of the colorectal stent continues to be defined. We report our experience in using colorectal stenting in managing malignant LBO.
Our overall technical success rate of 85% is comparable with the literature [7]. Our procedure duration of 82 minutes compares favorably with the reported duration of 114 minutes [7], as does our clinical success rate of 83%, with 53% to 86% reported elsewhere [5, 7]. The variation in clinical success rate may be due to local expertise and approach used. In our center, most stents are inserted under radiological guidance with selective endoscopic input. Others have described a different process, with all stents inserted endoscopically under fluoroscopic control [8]. This highlights how the pathway is hospital-specific and depends on local arrangements and facilities. Degree of obstruction was not systematically assessed endoscopically and therefore we could not comment on whether there is an association between degree of obstruction and likelihood of stent migration.
In fact, our own practice has evolved since the study period. We now perform endoscopic insertion with selective fluoroscopic assistance, the latter usually performed by an interventional radiologist. This change in our practice followed the acquisition of endoscopes that can deliver stents through a wider channel, compared to delivering the stent over a wire placed adjacent to the endoscope. Our practice now is therefore to place through stents through the endoscope for all but the most distal rectosigmoid lesions.
The high complication rates reported in the literature have led some to recommend that colorectal stents should not be used in the palliative setting, especially in patients who might otherwise benefit from palliative chemotherapy, which could be delayed by a complication [4]. In our experience, 3 patients suffered a stent-related perforation, which carries a high mortality. However, our overall stent-related complication rate of 21% compares well with 22% to 49% in the literature [5, 9]. Almost 2/3 of our patients avoided a stoma, which itself may expedite access to chemotherapy if required. As a bridge to surgery, colorectal stenting has been shown to allow a primary anastomosis rate of 70% and reduce stoma rate to only 26%, compared to only 30% and 68%, respectively, if emergency surgery is performed [2]. In our bridge to surgery group, we achieved a primary anastomosis rate of 78%.
No significant differences in morbidity, mortality, recurrence or survival have been reported between stenting and surgery for malignant LBO [2, 5]. Therefore, though colorectal stenting is less invasive, a reduction in morbidity and mortality does not follow, and this may be related to stent-related complications.
There is some debate about oncological outcomes following stenting. There has been a suggestion of increased local recurrence after stent insertion in the curative bridge to surgery setting [10], especially if complicated by perforation [11]. However, no significant difference in recurrence has been demonstrated [2, 12, 13]. In fact, beneficial oncological effects have even been postulated. Comparing paired tissue samples, at endoscopic biopsy before surgery and then in the surgically resected specimen after surgery, expression of the biomarker of proliferation Ki-67 and of some growth factors were decreased, and upstream cell cycle inhibitors were increased [14].
There is little detail in the literature on the indications for colorectal stenting. From a technical perspective, malignant strictures on the left-side, not in the low rectum, and with a length of less than 4 cm are thought to yield a better technical success rate [10]. All of the lesions in our study were left-sided. In addition to these technical considerations, other factors influencing treatment decisions include patient comorbidities, cancer stage, local resources and expertise available.
In the current economic climate, cost-effectiveness is also an important consideration. In a decision analysis study on a hypothetical cohort of patients, stent as a bridge to surgery showed a $4,000 saving [15]. In contrast, in the palliative setting, a randomized controlled trial showed stenting to be more expensive than surgical decompression [16]. Quality of life is also a critical outcome, especially in these patients who have a short life expectancy. Improved quality of life after stenting has been reported at 2 weeks postprocedure and also at 1 year [17]. However, another study from the same center showed no difference in quality of life at 1-month postprocedure [16].
A limitation of our study is that only patients who had stents inserted were included, and the group of patients with LBO that were managed without the use of stents were not included. Comparing the management of LBO with and without stents has been previously studied and was outside the scope of this study. We did not include patients with benign colorectal strictures and others have reported a higher rate of stent migration in this group [18]. Another limitation is this is a single-center study. However, the aim was to describe our real-world experience in a single-center.
In summary, in our experience colorectal stents can be used effectively to manage malignant LBO. The majority can be inserted under radiological guidance with only selective endoscopic input. As a bridge to surgery, most patients can avoid emergency surgery and have a primary anastomosis. In the palliative setting, the complication rate is acceptable and 2/3 avoid a permanent stoma.
Fig. 1.
Stent placement. (A) Radiograph of a stent placed with endoscopic support. (B) Radiograph of failed attempt at stent insertion.
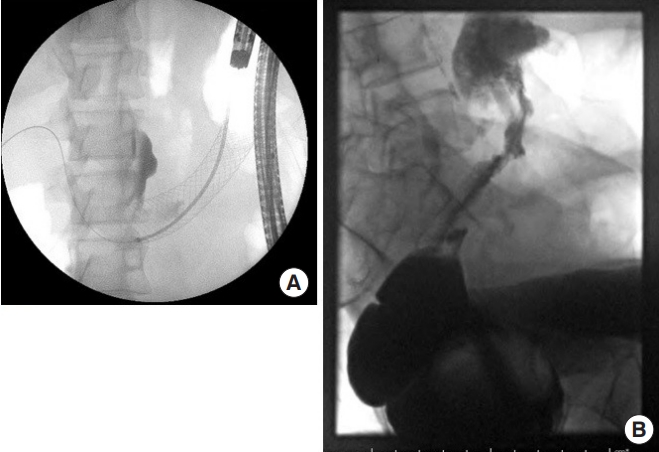
Table 1.
Demographic data and clinical outcomes
| Variable |
Treatment intent |
P-valuea | |
|---|---|---|---|
| Curative (bridge to surgery) | Palliative | ||
| No. of patients | 18 | 49 | - |
| Mean age (yr) | 71.5 | 77.6 | 0.11 |
| Male sex, n (%) | 10 (55.6) | 37 (75.5) | 0.11 |
| Mean comorbidity | 1.1 | 2.0 | < 0.05 |
| Metastatic disease, n (%) | 0 (0) | 31 (63.3) | < 0.001 |
| Surgery, n (%) | 18 (100) | 20 (40.8) | < 0.001 |
| Surgical procedure | High AR: 10 (3 open, 7 laparoscopic) | Colostomy: 14 | - |
| Open low AR+LI: 3 (all closed) | Loop ileostomy: 3 | ||
| Hartmann’s: 4 (3 open, 1 laparoscopic) | Hartmann’s: 2 | ||
| Extended right hemicolectomy: 1 | Laparotomy only: 1 | ||
| Permanent stoma rate, n (%) | 4 (22) | 19 (39) | 0.33 |
| Complication rate, n (%) | 2 (11) | 10 (20) | 0.38 |
| Mean length of stay (day) | |||
| Index admission | 8.9 | 12.7 | 0.26 |
| Total | 16.4 | 16.9 | 0.94 |
Table 2.
Endoscopic guidance and patient characteristics and outcomes
| Variable | Endoscopic guidance | Without endoscopic guidance | P-valuea |
|---|---|---|---|
| No. of patients | 24 | 49 | |
| Mean age (yr) | 78.7 | 74.8 | 0.27 |
| Male sex, n (%) | 10 (41.7) | 27 (55.1) | 0.28 |
| Procedure duration (hr) | 1.7 | 1.2 | < 0.05 |
| Technical failure, n (%) | 3 (12.5) | 7 (14.3) | 0.83 |
| Need for emergency surgery, n (%) | 7 (29.2) | 15 (30.6) | 0.92 |
REFERENCES
1. Mohd Suan MA, Tan WL, Soelar SA, Ismail I, Abu Hassan MR. Intestinal obstruction: predictor of poor prognosis in colorectal carcinoma? Epidemiol Health 2015;37:e2015017.



2. van den Berg MW, Sloothaak DA, Dijkgraaf MG, van der Zaag ES, Bemelman WA, Tanis PJ, et al. Bridge-to-surgery stent placement versus emergency surgery for acute malignant colonic obstruction. Br J Surg 2014;101:867–73.


3. Tomita M, Saito S, Makimoto S, Yoshida S, Isayama H, Yamada T, et al. Self-expandable metallic stenting as a bridge to surgery for malignant colorectal obstruction: pooled analysis of 426 patients from two prospective multicenter series. Surg Endosc 2019;33:499–509.


4. Fernandez-Esparrach G, Bordas JM, Giraldez MD, Gines A, Pellise M, Sendino O, et al. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol 2010;105:1087–93.


5. Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and metaanalysis. Surg Oncol 2013;22:14–21.


6. National Institute for Health and Care Excellence (NICE). Colorectal cancer: diagnosis and management. Clinical guideline [CG131] [Internet]. London, NICE; 2014 [cited 2020 Feb 10]. Available from: https://www.nice.org.uk/guidance/cg131
7. Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev 2011;(11):CD007378.

8. Mehmood RK, Parker J, Kirkbride P, Ahmed S, Akbar F, Qasem E, et al. Outcomes after stenting for malignant large bowel obstruction without radiologist support. World J Gastroenterol 2014;20:6309–13.



9. Blake P, Delicata R, Cross N, Sturgeon G, Hargest R. Large bowel obstruction due to colorectal carcinoma can be safely treated by colonic stent insertion: case series from a UK district general hospital. Colorectal Dis 2012;14:1489–92.


10. Sagar J. Role of colonic stents in the management of colorectal cancers. World J Gastrointest Endosc 2016;8:198–204.



11. Avlund TH, Erichsen R, Ravn S, Ciplys Z, Andersen JC, Laurberg S, et al. The prognostic impact of bowel perforation following self-expanding metal stent as a bridge to surgery in colorectal cancer obstruction. Surg Endosc 2018;32:328–36.



12. Ceresoli M, Allievi N, Coccolini F, Montori G, Fugazzola P, Pisano M, et al. Long-term oncologic outcomes of stent as a bridge to surgery versus emergency surgery in malignant left side colonic obstructions: a meta-analysis. J Gastrointest Oncol 2017;8:867–76.



13. Erichsen R, Horvath-Puho E, Jacobsen JB, Nilsson T, Baron JA, Sørensen HT. Long-term mortality and recurrence after colorectal cancer surgery with preoperative stenting: a Danish nationwide cohort study. Endoscopy 2015;47:517–24.


14. Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Kawano Y, Yamahatsu K, et al. Colonic stent-induced mechanical compression may suppress cancer cell proliferation in malignant large bowel obstruction. Surg Endosc 2019;33:1290–7.



15. Targownik LE, Spiegel BM, Sack J, Hines OJ, Dulai GS, Gralnek IM, et al. Colonic stent vs. emergency surgery for management of acute left-sided malignant colonic obstruction: a decision analysis. Gastrointest Endosc 2004;60:865–74.


16. Young CJ, Zahid A. Randomized controlled trial of colonic stent insertion in non-curable large bowel obstruction: a post hoc cost analysis. Colorectal Dis 2018;20:288–95.



- TOOLS
-
METRICS

-
- 4 Web of Science
- 0 Crossref
- Scopus
- 2,072 View
- 83 Download
- Related articles in ACP