- Search
| Ann Coloproctol > Volume 37(5); 2021 > Article |
|
Abstract
Purpose
Proctectomy for the treatment of rectal cancer results in inevitable changes to bowel habits. Symptoms such as fecal incontinence, constipation, and tenesmus are collectively referred to as low anterior resection syndrome (LARS). Among the several risk factors that cause LARS, anastomotic leakage (AL) is a strong risk factor for permanent stoma formation. Therefore, the purpose of this study was to investigate the relationship between the severity of LARS and AL in patients with rectal cancer based on the LARS score and the Memorial Sloan Kettering Cancer Center (MSKCC) defecation symptom questionnaires.
Methods
We retrospectively analyzed patients who underwent low anterior resection for rectal cancer since January 2010. Patients who completed the questionnaire were classified into the AL group and control group based on medical and imaging records. Major LARS and MSKCC scores were analyzed as primary endpoints.
Results
Among the 179 patients included in this study, 37 were classified into the AL group. After propensity score matching, there were significant differences in the ratio of major LARS and MSKCC scores of the control group and AL group (ratio of major LARS: 11.1% and 37.8%, P < 0.001; MSKCC score: 67.29┬▒10.4 and 56.49┬▒7.2, respectively, P < 0.001). Univariate and multivariate analyses revealed that AL was an independent factor for major LARS occurrence and MSKCC score.
Surgical resection is the basic principle of rectal cancer treatment. With advances in surgical techniques, such as total mesorectal excision, chemotherapy, and radiation therapy, survival rates for rectal cancer have gradually increased, with a 5-year survival rate exceeding 70% in Korea [1].
However, proctectomy involves inevitable changes in bowel habits, which can impede quality of life. After rectal resection, a series of symptoms occur, including fecal incontinence, constipation, tenesmus, urgency, feeling of incomplete emptying, and frequent bowel movement that are collectively referred to as low anterior resection syndrome (LARS) [2, 3]. The incidence of LARS varies across studies but is commonly reported in more than half of the patients with rectal resection. According to a cohort study of 961 patients who underwent rectal resection in Denmark, LARS occurs in 64% of patients with rectal resection; of which, 41% complained of severe symptoms [4]. LARS symptoms were alleviated 6 months to 1 year postoperatively [2, 5]. However, there are reports that about 50% of patients complain of symptoms, even 15 years postoperatively [6, 7]. As such, LARS can affect the quality of life over a long period of time.
Various factors affect the incidence and severity of LARS. Representative risk factors include preoperative radiotherapy, anastomosis near the anal verge, end-to-end anastomosis without a pouch, and anastomotic leakage (AL) [3]. Of these, AL is a complication reported in about 20% of patients who underwent endto-end anastomosis after rectal resection [8, 9] and is a strong risk factor for permanent stoma formation [10]. Nevertheless, due to patientsŌĆÖ rejection of permanent stoma or a burden on the operator, the operator maintains the continuity of the intestinal tract, except in inevitable cases, such as sepsis due to AL. However, from a long-term perspective, this can worsen the quality of life of patients.
Several studies have been conducted on AL associated with LAR, and methods to prevent this have also been demonstrated [9, 11]. However, few studies have investigated the association between AL and LARS, and especially in Korea, even though the incidence of LARS has not been properly investigated [12]. There are no clinical studies that prove inflammatory reactions, such as fibrotic scar or chronic sinus, induced by AL cause deterioration of the remnant rectum and lead to LARS. If the association between LARS and AL is proven through research, the rationale for this would be that preventing AL can prevent the occurrence of LARS. Therefore, the purpose of this study was to investigate the relationship between AL and the severity of LARS in patients with rectal cancer.
Patients who underwent low anterior resection for rectal cancer from January 2010 to September 2019 at Yeungnam University Medical Center in Daegu, Korea were reviewed retrospectively. Low anterior resection was performed by an experienced colorectal surgeon. At the time of investigation, the study was performed on patients who underwent surgery more than 1 year before. The exclusion criteria were (1) permanent or temporary ostomy at the time of investigation, (2) patients who underwent colon resection or small bowel resection of > 100 cm or gastrectomy in addition to low anterior resection, (3) patients with cognitive or mental disabilities who lack the ability to understand the questionnaire and respond properly, and (4) patients who disagree with the study or have lost contact with the study investigators. If patients did not visit the hospital after follow-up was completed, the researchers explained the study details through telephonic conversations, and if patients agreed, they could visit the hospital at the desired time and complete the questionnaire.
The Korean version of the LARS score questionnaire [13] was used, and the scores were converted according to each item. The sum of the scores of each item was classified as follows: 0 to 20, no LARS; 21 to 29, minor LARS; 30 to 42, major LARS. The items of the Memorial Sloan Kettering Cancer Center (MSKCC) [14] questionnaire were translated to Korean. The scores of the 1st, 4th, 5th, 7th, 11th, and 12th items were recalculated because a higher score represents a more severe defecation problem. We evaluated the defecation symptom as the sum of the scores for each item, indicating that the higher the score, the better the defecation function.
In this study, AL is defined as defects in the intestinal wall of the anastomosis site, and determined based on medical and imaging records and classified as follows based on the classification system commonly used in clinical practice [15]: grade A leakage requires no therapeutic intervention, and does not affect a patientŌĆÖs management; grade B leakage requires active therapeutic intervention, but is manageable without reoperation; and grade C leakage requires reoperation. Clinically, AL signs were defined as fever, abdominal pain, fecal discharge from a drain, peritoneal irritation sign, and pelvic abscess postoperatively. All clinically diagnosed ALs were confirmed by digital rectal examination or computed tomography. The medical records of patients who completed the questionnaire were analyzed retrospectively and classified into the AL group (grade B, C) and control group (no AL, grade A).
To minimize the effect of confounders on selection bias, propensity score and nearest-neighbor matching (PSM) analyses were performed. Patients in the AL group were matched on a 1:2 propensity score with patients in the control group according to age, sex, body mass index, tumor location, neoadjuvant treatment, surgical approach, operative method, anastomosis type, and fecal diversion.
Baseline demographics were compared between the AL and control groups. The Student t-test or Mann-Whitney U-test was used for continuous variables; and for categorical variables, a chi-square test or Fisher exact test was used. Univariate analyses were performed to identify factors associated with the LARS and MSKCC total scores by using logistic regression and linear regression analyses. Variables with P-values of < 0.05 in univariate analyses were included in the multivariate analyses. All statistical analyses were considered significant at P < 0.05. Statistical analysis was performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA).
This study was approved by the Institutional Review Board of Yeungnam University Medical Center (No. YUMC 2020-03-117-007) with the written informed consent from the patients.
A survey including the LARS score and MSKCC defecation symptom questionnaires was conducted between November 2019 and September 2020. A total of 208 patients met the selection criteria. Patients who were scheduled to visit our institution for the treatment or follow-up of rectal cancer answered the questionnaire after the explanation of the researcher. Of the 208 patients, 179 answered the questionnaire (Fig. 1).
Among the 179 patients, 23 patients (12.8%) had grade B leakage and 14 (7.8%) had grade C leakage. A total of 37 patients with grade B and C leakage were classified into the AL group and the remaining 142 patients were classified into the control group.
There was no difference in baseline characteristics between the 2 groups (Table 1). By performing PSM, 74 out of 142 patients in the control group were matched. There was no difference between the 2 groups except for the time to surgery factor after PSM. Before and after PSM, the LARS and MSKCC scores were significantly different between the control group and AL group (LARS score [before matching]: 14.60 ┬▒ 12.6 and 28.30 ┬▒ 6.4, P < 0.001; LARS score [after matching]: 15.62 ┬▒ 13.0 and 28.30 ┬▒ 6.4, P < 0.001; MSKCC score [before matching]: 66.88 ┬▒ 9.1 and 55.24 ┬▒ 7.0, P < 0.001; MSKCC score [after matching]: 66.63 ┬▒ 8.5 and 55.24 ┬▒ 7.0, P < 0.001). Moreover, the ratio of major LARS was significantly higher in the AL group (control group and AL group; before matching: 12.7% and 37.8%, P < 0.001; after matching: 13.5% and 37.8%, P < 0.001).
Before PSM, univariate analysis showed that major LARS was associated with neoadjuvant therapy, tumor stage, and grade B and C AL. After PSM, neoadjuvant therapy, fecal diversion, tumor stage, and grade B and C AL were significant factors with major LARS in the univariate analysis (Table 2). Multivariate analysis demonstrated that grade B and C AL and neoadjuvant therapy were independent factors for major LARS (Table 3).
The same analysis was conducted to identify the independent factor for the MSKCC score. Before PSM, grade B and C AL were independent factors for MSKCC score in univariate analysis. Sex, tumor location, and grade B and C AL were independent factors for MSKCC in the univariate analysis after PSM (Table 4). The multivariate analysis showed that sex, tumor location, and grade B and C AL were significantly associated with MSKCC score (Table 5).
Our study findings showed that AL and neoadjuvant chemoradiotherapy affected LARS and defecation symptoms in patients with rectal cancer who underwent LAR. These results are comparable to those of previous studies that also evaluated the factor which affect LARS and defecation symptoms [4, 16, 17].
The exact diagnosis and approach to LARS depend on asking appropriate questions about the patientŌĆÖs symptoms. According to a recent meta-analysis, due to the lack of a LARS definition, long-term bowel function was not evaluated, and in 65% of studies, a validated questionnaire was not used [18]. The surveys for investigating the quality of life that have been published are very diverse and complex; therefore, it takes a long time to complete and analyze them. It has also been reported that they represent specific symptoms and are not suitable for collecting complex symptoms associated with bowel movements and reflecting the quality of life associated with bowel movements [19].
According to several studies, there are a number of factors that worsen LARS, such as radiotherapy, the extent of rectal excision, the creation of a colonic pouch, and AL. In this study, we focused on AL, a deteriorating factor of LARS. Since there are many factors that affect LARS among the risk factors of AL, such as low-lying tumor and preoperative chemoradiation, correction between the leak group and the no-leak group was performed through PSM. Regarding the impact of postoperative AL on defecation symptoms, there are not much available data and a somewhat heterogeneous investigation of symptoms. There are also few studies on the histological approach of AL. Daams et al. [20] showed that the healing of gastrointestinal anastomosis in an experimental model occurred by the formation of a fibrotic cap at the serosal portion, which formed a matrix for fibroblasts. Based on this result, AL occurrence is considered a negative event for bowel function because of the inflammatory change and excessive fibrotic scarring that may develop thereafter in the pelvic cavity. This can alter compliance and the capacity of the neorectum, which can induce urgency or incontinence.
If neoadjuvant therapy was performed, surgery was performed 6 weeks after the end of neoadjuvant therapy. Therefore, it can be seen that LARS is worsened by ischemia and fibrosis caused by progressive obliterating endarteritis and the late toxicity of radiotherapy. Gastrointestinal tract ulceration causes symptoms such as perforation, fistulization, and peritonitis, and is associated with an extensive area of fibrosis [21]. Anal sphincter damage is also induced by radiotherapy, which is due to the damage to the myenteric plexus and smooth-muscle hypertrophy [22]. The length of the residual rectum on magnetic resonance imaging affects LARS severity, and it is reported that LARS severity is high when the length of the residual rectum is less than 4 cm [23].
Reduced neorectal reservoir volume is considered a major cause of urgency or incontinence. According to several studies, low-lying tumors or anastomoses of < 5 cm from the anal verge are independent risk factors for deteriorated defecation symptoms [17,23]. Damage to the internal anal sphincter during rectal mobilization causes passive incontinence [24], and damage to the pelvic floor innervations leads to fecal incontinence and urgency [25]. Moreover, a decrease in the length of the urethral rectum leads to a decrease in neorectal capacity, which leads to a worsening of bowel dysfunction [26].
In our study, female patients showed better bowel function than male patients. To date, there have been no studies that have studied the relationship between bowel dysfunction after low anterior resection and sex difference. The result of our study is expected to be due to the anatomical difference of the pelvis, which is related to the difficulty of surgery. Females had a significantly longer pelvic inlet and outlet, while males had a greater pelvic depth [27]. Therefore, female patients will experience less nerve damage during surgery, and bowel function postoperatively is expected to be better than that of male patients. However, a larger study is needed to confirm this.
In addition to loop ileostomy, which is the traditional method of fecal diversion, patients who performed fecal diversion using the FDD [28], which is being clinically tested at our hospital, are included. In the clinical trial at that time, it was concluded that the ratio of AL between the patient group who underwent ileostomy and the patient group using FDD was the same; hence, it was decided to include patients who used FDD in this study. As was mentioned in the results section, there was no significant difference in LARS score and MSKCC score between the FDD group and the loop ileostomy groups. Therefore, FDD and loop ileostomy were considered as the same fecal diversion method when performing univariate and multivariate analyses.
This study has several limitations. First, it is a nonrandomized study design. Operators must do their best to prevent AL occurrence, and there cannot be a study design that randomizes AL occurrence. Since patients were classified into 2 groups according to the presence or absence of AL, selection bias may occur regarding factors that may cause AL. Thus, the author implemented the PSM method to compensate for the selection bias. For more effective matching, the number of patients should be greater than in this study. Therefore, prospective multicenter research is needed. Second, this is a study based on a survey; since the questionnaire survey is conducted based on the subjective symptoms of the patient, it may be difficult to use it as an objective indicator, and as the survey is mainly conducted on elderly patients, it is difficult to expect accurate memories. Third, the time of the questionnaire survey from surgery was different for each patient. Since LARS shows a trend of improvement from 1 year postoperatively, studies were conducted on patients who underwent operation for more than 1 year, but many studies have shown that symptoms persist for up to 2 years postoperatively. Therefore, it is necessary to investigate defecation symptoms or LARS at several time points, not at 1-time point postoperatively.
In conclusion, this study showed that AL is a risk factor for major LARS and changes in defecation function after low anterior resection and neoadjuvant chemoradiotherapy. Further prospective multicenter studies are needed to confirm the negative prognostic factors of AL and the relationship with major LARS.
Fig.┬Ā1.
Flow chart of study patients. LAR, low anterior resection; uLAR, ultra low anterior resection; AL, anastomotic leakage.
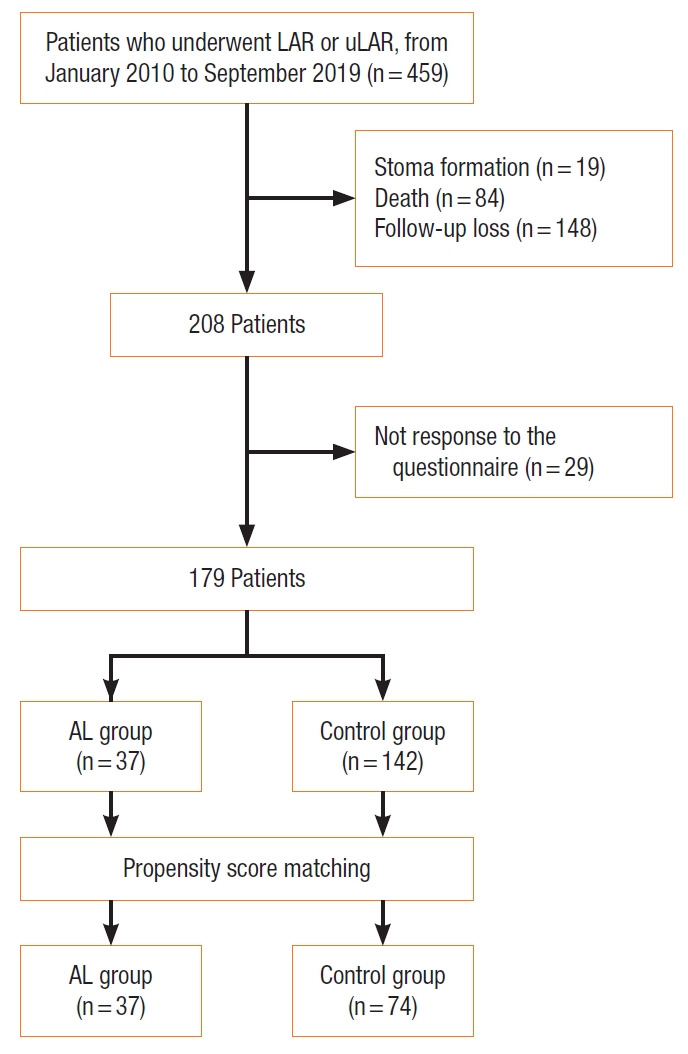
Fig.┬Ā2.
Graph of correlation between low anterior resection syndrome (LARS) score and Memorial Sloan Kettering Cancer Center (MSKCC) score. (A) Before propensity score matching (PSM). (B) After PSM.

Table┬Ā1.
Comparison of characteristics before and after propensity score matching
Values are expressed as mean┬▒standard deviation or number (%).
AL, anastomotic leakage; BMI, body mass index; LAR, low anterior resection; uLAR, ultra low anterior resection; FDD, fecal diversion device; IMA, inferior mesenteric artery; LARS, low anterior resection syndrome; MSKCC, Memorial Sloan Kettering Cancer Center.
Table┬Ā2.
Univariate analysis of risk factors for major LARS
Table┬Ā3.
Multivariate analysis of risk factors for major LARS
Table┬Ā4.
Univariate analysis of risk factors for MSKCC score
Table┬Ā5.
Multivariate analysis of risk factors for MSKCC score
Table┬Ā6.
Comparison of LARS score and MSKCC score according to fecal diversion method
REFERENCES
1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nik┼Īi─ć M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023ŌĆō75.


2. Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol 2012;13:e403ŌĆō8.


3. Sarcher T, Dupont B, Alves A, Menahem B. Anterior resection syndrome: what should we tell practitioners and patients in 2018? J Visc Surg 2018;155:383ŌĆō91.


4. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922ŌĆō8.

5. Martellucci J, Sturiale A, Bergamini C, Boni L, Cianchi F, Coratti A, et al. Role of transanal irrigation in the treatment of anterior resection syndrome. Tech Coloproctol 2018;22:519ŌĆō27.


6. Chen TY, Wiltink LM, Nout RA, Meershoek-Klein Kranenbarg E, Laurberg S, Marijnen CA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer 2015;14:106ŌĆō14.


7. Sturiale A, Martellucci J, Zurli L, Vaccaro C, Brusciano L, Limongelli P, et al. Long-term functional follow-up after anterior rectal resection for cancer. Int J Colorectal Dis 2017;32:83ŌĆō8.


8. Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H, et al. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget 2015;6:36884ŌĆō93.



9. Zhang W, Lou Z, Liu Q, Meng R, Gong H, Hao L, et al. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int J Colorectal Dis 2017;32:1431ŌĆō7.


10. Kim MJ, Kim YS, Park SC, Sohn DK, Kim DY, Chang HJ, et al. Risk factors for permanent stoma after rectal cancer surgery with temporary ileostomy. Surgery 2016;159:721ŌĆō7.


11. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 2013;257:665ŌĆō71.

12. Ihn MH, Kang SB, Kim DW, Oh HK, Lee SY, Hong SM. Risk factors for bowel dysfunction after sphincter-preserving rectal cancer surgery: a prospective study using the Memorial Sloan Kettering Cancer Center bowel function instrument. Dis Colon Rectum 2014;57:958ŌĆō66.

13. Kim CW, Jeong WK, Son GM, Kim IY, Park JW, Jeong SY, et al. Validation of Korean version of Low Anterior Resection Syndrome Score Questionnaire. Ann Coloproctol 2020;36:83ŌĆō7.



14. Temple LK, Bacik J, Savatta SG, Gottesman L, Paty PB, Weiser MR, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum 2005;48:1353ŌĆō65.


15. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339ŌĆō51.


16. Wells CI, Vather R, Chu MJ, Robertson JP, Bissett IP. Anterior resection syndrome: a risk factor analysis. J Gastrointest Surg 2015;19:350ŌĆō9.



17. Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, et al. Predicting the risk of bowel-related quality-of-life impairment after restorative resection for rectal cancer: a multicenter cross-sectional study. Dis Colon Rectum 2016;59:270ŌĆō80.

18. Scheer AS, Boushey RP, Liang S, Doucette S, OŌĆÖConnor AM, Moher D. The long-term gastrointestinal functional outcomes following curative anterior resection in adults with rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2011;54:1589ŌĆō97.


19. Chen TY, Emmertsen KJ, Laurberg S. What are the best questionnaires to capture anorectal function after surgery in rectal cancer? Curr Colorectal Cancer Rep 2015;11:37ŌĆō43.


20. Daams F, Monkhorst K, van den Broek J, Slieker JC, Jeekel J, Lange JF. Local ischaemia does not influence anastomotic healing: an experimental study. Eur Surg Res 2013;50:24ŌĆō31.


21. Carr ND, Pullen BR, Hasleton PS, Schofield PF. Microvascular studies in human radiation bowel disease. Gut 1984;25:448ŌĆō54.



22. Varma JS, Smith AN, Busuttil A. Function of the anal sphincters after chronic radiation injury. Gut 1986;27:528ŌĆō33.



23. Beppu N, Kimura H, Matsubara N, Tomita N, Yanagi H, Yamanaka N. Long-term functional outcomes of total mesorectal excision following chemoradiotherapy for lower rectal cancer: stapled anastomosis versus intersphincteric resection. Dig Surg 2016;33:33ŌĆō42.


24. Machado M, Nygren J, Goldman S, Ljungqvist O. Similar outcome after colonic pouch and side-to-end anastomosis in low anterior resection for rectal cancer: a prospective randomized trial. Ann Surg 2003;238:214ŌĆō20.


25. Wallner C, Lange MM, Bonsing BA, Maas CP, Wallace CN, Dabhoiwala NF, et al. Causes of fecal and urinary incontinence after total mesorectal excision for rectal cancer based on cadaveric surgery: a study from the Cooperative Clinical Investigators of the Dutch total mesorectal excision trial. J Clin Oncol 2008;26:4466ŌĆō72.


26. Matzel KE, Stadelmaier U, Muehldorfer S, Hohenberger W. Continence after colorectal reconstruction following resection: impact of level of anastomosis. Int J Colorectal Dis 1997;12:82ŌĆō7.










