- Search
| Ann Coloproctol > Volume 38(4); 2022 > Article |
|
Abstract
Purpose
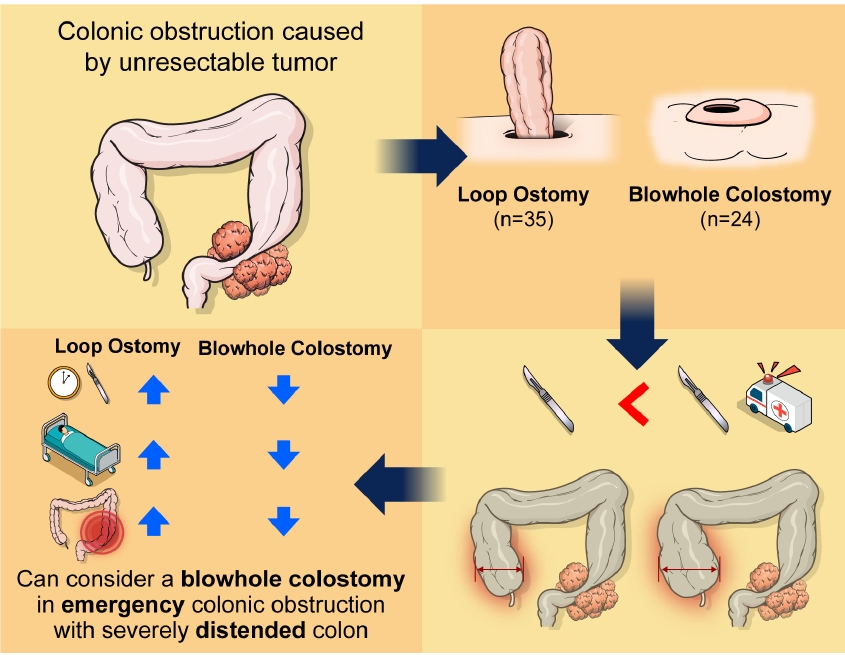
Surgery to create a stoma for decompression might be required for unresectable stage IV cancer patients with complete colonic obstruction. The aim of this study was to compare the results of blowhole colostomy with those of loop ostomy.
Methods
Palliative ileostomy or colostomy procedures performed at a single center between January 2011 and October 2020, were analyzed retrospectively. Fifty-nine patients were identified during this period. The demographic characteristics and outcomes between the blowhole colostomy group (n=24) and the loop ostomy group (n=35) were compared.
Results
The median operative time tended to be shorter in the blowhole colostomy group (52.5 minutes; interquartile range [IQR], 43–65) than in the loop ostomy group (60 minutes; IQR, 40–107), but the difference did not reach statistical significance (P=0.162). The median length of hospital stay was significantly shorter with blowhole colostomy (blowhole, 13 days [IQR, 9–23]; loop, 21 days [IQR, 14–37]; P=0.013). Mean cecum diameter was significantly larger in the blowhole group than in the loop group (8.83±1.91 cm vs. 6.78±2.36 cm, P=0.001), and the emergency operation rate was higher in the blowhole group than in the loop group (22 of 24 [91.7%] vs. 23 of 35 [65.7%], P=0.021).
Graphical Abstract
Large bowel obstruction can be caused by a variety of etiologies, such as congenital anomalies, inflammatory bowel disease, cancer of the digestive system or adjacent organs (e.g., ovarian, vaginal, prostate), and metastatic cancer [1-8]. Acute complete colonic obstruction is an abdominal emergency that requires urgent surgery or other intervention. If left untreated, the signs and symptoms of obstruction worsen, and marked distension of the large intestine proceeds [3-15]. Colonic obstruction can lead to edema of the bowel mucous membrane, ischemia of the intestine, and bowel infarction and perforation [15, 16]. This condition can be fatal in patients with unresectable end-stage cancer who have poor nutritional support and a poor general condition [11-13, 17].
Surgery has been the primary treatment for malignant colonic obstruction for decades, although the postoperative morbidity and mortality rates are higher in emergency situations than for elective surgery [1, 6-11]. For this reason, endoscopic self-expandable metal stents are being investigated as an alternative treatment for these patients. Nevertheless, colonic stents might not be placed due to evidence of clinical failure in cases with severe adhesions after laparotomy, severe symptoms related to intestinal obstruction or fistula formation in multiple areas, or aggressive tumor invasion [2, 7, 9, 12]. Depending on the lesion, stenting often is not appropriate, and there is a risk of stent migration or bowel perforation [6-11]. Furthermore, stent placement might not be feasible in hospitals with limited resources [8]. Therefore, these patients might require emergency surgical procedures such as stoma creation or bypass surgery for fecal diversion and decompression to relieve colon obstruction [3, 5, 18].
Ostomy for palliation can be performed in the sigmoid colon, transverse colon, cecum, or ileum [19-21]. The location of the stoma is chosen based on the site of obstruction and patient condition [4, 11-14, 17, 18, 20-24]. However, there is no general consensus with regard to the optimal type of stoma [4, 13, 18-21, 25, 26]. Usually, loop type ostomy is preferred; however, when massive distention is present, there is an increased likelihood of difficulty in placing the maturing loop [1, 19, 27, 28]. In such situations, blowhole type colostomy can be an alternative procedure [1, 2].
Blowhole colostomy was introduced by Dr. Rupert Turnbull at the Cleveland Clinic in 1953, as an emergency treatment for bowel decompression in toxic colitis patients [1, 2, 25, 27]. These patients had toxic megacolon secondary to inflammatory bowel disease and Clostridium difficile colitis [16, 27, 28]. This technique was performed as a bridge therapy before colectomy in patients at risk of perforation due to thinning of the colon wall or in patients at high risk for resection [1, 16, 25, 27]. A blowhole colostomy and ileostomy were performed in a female patient who developed severe toxic colitis during pregnancy [28]. She gave birth successfully, and an interval ileal pouch-anal construction was performed successfully. Particularly in elderly terminal cancer patients with an American Society of Anesthesiology (ASA) physical status classification of III or IV, high morbidity and mortality can occur depending on the surgical approach due to comorbid risk factors. Blowhole colostomy also has been created in patients with malignant bowel obstruction to avoid manipulating the colon and to maintain the patient during the critical phase [1, 2]. The aim of this study was to compare the surgical results of blowhole colostomy and loop ostomy in terminal cancer patients with urgent or emergent complete colonic obstruction.
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (No. KSH 2021-02-026), and written informed consent was obtained for publication of the accompanying clinical images.
A chart review was performed retrospectively for all patients who underwent colostomy or ileostomy from January 2011 to October 2020, at a single tertiary referral center. Among these patients, those with malignant large bowel obstruction caused by unresectable primary, recurrent, or metastatic malignant tumor were identified for inclusion. Colonic obstruction was confirmed in the presence of extensive abdominal distension based on clinical symptoms, vital signs, blood laboratory tests, and physical examination of the patient and was diagnosed through imaging studies, such as abdominal X-ray and computed tomography (CT) scan. Patients who had a colonic mass that caused colonic obstruction or who underwent end type colostomy were excluded from our analysis.
The patients were divided into 2 groups depending on blowhole colostomy or loop ostomy and were evaluated for the following factors: age, sex, body mass index, malnutrition, ASA physical status classification, cecum diameter, emergent operation rate, total operative time, time to liquid or regular diet, length of hospital stay, 30-day or in-hospital mortality, and rates of complications. Patient demographics and perioperative clinical parameters were collected from an electronic medical record system.
If the surgery took place within 12 hours of diagnosis, it was defined as an emergency operation; if it was performed more than 12 hours or the next day after diagnosis, it was defined as an elective operation. Operative time was defined as the total minutes from the first incision to placement of the final suture. Postoperative morbidity and mortality rates were investigated for more than 3 months or until the patient’s death. Postoperative morbidity was defined as any complication that required additional treatment, prolonged hospital stay, or frequent outpatient clinic visits.
The primary outcome measure was clinically successful colonic decompression, defined as relief of obstructive symptoms and the ability to pass flatus and stool after oral intake. The secondary outcome measure was surgical outcome by group (blowhole colostomy vs. loop ostomy).
A blowhole colostomy was placed in the cecum (n= 13) or transverse colon (n= 11) (Fig. 1A, B). After careful review of the abdominopelvic CT images, it was confirmed that the diameter of the cecum or transverse colon increased by more than 9 cm, and the abdominal wall was not thicker than 2 cm (Fig. 2). A skin incision was made in the right lower quadrant (McBurney’s point) for the cecum or in the upper abdomen through the rectus abdominis muscle for the transverse colon. An approximately 4-cm transverse skin incision was carried from the skin to the fascia and then down to the peritoneum. Upon entry into the abdomen, the colon wall was inspected to identify necrosis or perforation, and the best site to perform colostomy was determined. If there was a suspected area of necrosis, a stoma was created including this area, and the core of necrosis was removed when the colon was opened and decompressed. The cecum was sutured continuously to the peritoneum and external oblique aponeurosis, or the transverse colon was sutured to the peritoneum and the anterior fascia of the rectus abdominis muscle using 4-0 sutures and fixed. At this time, a fine needle was used to prevent stool leakage, and the interval between each stitch was about 3–4 mm to prevent stool from entering the abdominal cavity when the colon was opened. After fixing the colon, a 4-cm horizontal incision was made in the anterior wall of the colon, and any feces or gas were suctioned. Then, continuous or intermittent sutures were placed in the epidermal layer using 4-0 Vicryl suture, and an appropriate stoma bag was attached.
Loop type stoma was created in the ileum (n= 16), transverse colon (n= 7), or sigmoid colon (n= 12). After careful review of the abdominopelvic CT images, an upper abdominal incision was extended down to the fascia at the location of maximal transverse colon dilation for transverse colostomy. If the sigmoid colon and ileum were involved, a lower abdominal incision was made in the rectus abdominis muscle. The bowel was delivered through the abdominal wall. The previously placed suture or mark in the distal segment was used to confirm that the bowel was viable and had not twisted. An ostomy rod was placed through the mesentery in standard fashion before maturation of the ostomy via standard means, and then an appropriate stoma bag was attached.
Statistical analysis was performed using IBM SPSS Statistics ver. 24 (IBM Corp., Armonk, NY, USA). Continuous variables were compared using Student t-test or Mann-Whitney U-test, and categorical variables were analyzed using the chi-square test or Fisher exact test. The P-values of < 0.05 were considered significant.
Between January 2011 and October 2020, 59 patients underwent blowhole colostomy (n= 24) or loop ostomy (n= 35) creation due to complete colonic obstruction. The patients who underwent blowhole colostomy demonstrated a significantly greater proportion of stomach cancer compared with the loop ostomy patients in this evaluation of the obstruction etiology (P= 0.037). However, there was no significant difference in other etiology between the 2 groups. The characteristics of the patients in the groups are summarized in Table 1. No difference in age, sex, body mass index, albumin, or ASA physical status classification was noted. However, mean cecum diameter was significantly larger in the blowhole group than in the loop group (8.83± 1.91 cm and 6.78± 2.36 cm, respectively) (P= 0.001), and the emergency operation rate was higher in the blowhole group than in the loop group (22 of 24 [92.0%] vs. 23 of 35 [65.7%], P= 0.021). Ileostomy was more often performed in the loop group, while cecostomy was more prevalent in the blowhole group (16 of 35 [45.7%] vs. 13 of 24 [54.1%], P< 0.001).
The surgical outcomes are shown in Table 2. The median operative time tended to be shorter in the blowhole colostomy group (52.5 minutes; interquartile range [IQR], 43–65) than in the loop ostomy group (60 minutes; IQR, 40–107), but the difference did not reach statistical significance (P= 0.162). The median length of hospital stay was significantly shorter with blowhole colostomy (blowhole, 13 days [IQR, 9–23]; loop, 21 days [IQR, 14–37], P= 0.013). All patients resumed a normal oral intake, and their obstructive symptoms resolved except for 2 patients who died on the 1st postoperative day due to sepsis. There were no differences in time to resuming a liquid or regular diet and 30-day or in-hospital mortality between the 2 groups.
Surgical complications occurred more frequently in the loop ostomy group than in the blowhole colostomy group (12 [34.3%] vs. 6 [25.0%], respectively), but the difference did not reach statistical significance (P= 0.447). Major surgical complications, which were defined as grade III or IV according to the Clavien-Dindo classification system, occurred in 5 patients (41.7%) in the loop ostomy group and 6 patients (100%) in the blowhole colostomy group. This difference was statistically significant (P= 0.038). Stoma-related complications were reported more frequently in the loop ostomy group (8 of 35, 22.9%) than in the blowhole colostomy group (5 of 24, 20.8%), but the difference did not reach statistical significance (P> 0.999). The rate of stoma-related minor surgical complications was higher in the loop ostomy group (5 of 35, 14.3%) compared to the blowhole colostomy group (0 of 24, 0%). However, the number of stoma-related major complications was higher in the blowhole group (5 of 24, 20.8%) than in the loop group (3 of 35, 8.6%) (Table 3).
This study confirmed that blowhole colostomy can be an appropriate emergency surgical method compared to loop ostomy. In addition, blowhole colostomy is associated with a shorter length of hospital stay and fewer postoperative complications, which can improve the quality of life in terminal cancer patients. This study showed that 91% of the blowhole type group underwent emergency surgery. Their complication rate was 25%, which was not statistically significant compared to the loop type but showed a relatively low tendency. Although the incidence of major complications requiring intervention in the blowhole group was significantly higher, minor complications were not observed.
To our knowledge, this was the first study to compare the blowhole colostomy procedure with loop ostomy as a type of palliation. Traditionally, surgical management of acute bowel obstruction involves a fecal diversion or resection, which carries a perioperative morbidity as high as 46% and an overall mortality as high as 28% for each procedure [6-10, 12]. When creating an ostomy in terminal cancer patients with an unresectable colonic obstruction, a loop ileostomy or colostomy typically is required for both fecal diversion and decompression [3-5, 13, 17, 18, 22, 29]. In contrast, the blowhole type of colostomy does not completely divert the fecal flow. This approach is inappropriate if inflammation is present in the abdominal cavity or there is leakage of the anastomosis. Blowhole colostomy can be considered first when the cecum or transverse colon has massive distension, and the subcutaneous fat layer of the abdominal wall is not thick. It can be also applied to situations in which colonic decompression cannot be efficiently performed due to difficulty in maturation in loop type stoma creation. On the other hand, the loop ostomy tends to be preferred when the flow of feces is preferentially required rather than emergency colonic decompression in complete obstruction. Since the cecum is the area of the colon with the widest diameter, the pressure on the intestinal wall when the distal colon is obstructed is highest here according to Laplace’s law [1, 2]. Additionally, if the cecum diameter is larger than 9 cm, the likelihood of bowel ischemia and perforation increases [15, 16, 30]. Therefore, when blowhole colostomy is performed in the cecum, it is more effective at decompressing the colon. In addition, it is possible to prevent bowel perforation by identifying the site suspected of necrosis, removing this area and creating a stoma including this area. As described in this study, it is more efficient to perform blowhole colostomy with a relatively shorter operation time and a relatively low probability of surgical complications in these patients. This procedure is cost-effective because the length of hospital stay is short. Furthermore, Kuk et al. [2] showed spontaneous closure of the cecum stoma in 10% of patients when the fecal flow was restored due to reduction in the size of the tumor at the intestinal obstruction after chemo-radiation therapy. Similarly, 1 case of spontaneous closure was observed with blowhole colostomy performed on a patient with recurrent gastric cancer at our institution, as obstruction improved after chemotherapy (Fig. 1C).
In this study, all 59 patients underwent emergent or elective surgery. One patient had been previously treated with a palliative stent, but complete obstruction still occurred due to an ingrowing malignant mass on the rectum. Another patient had colocolic intussusception with complete colonic obstruction due to advanced descending colon cancer, which has a high risk of perforation when a stent is inserted. In both cases, a blowhole colostomy was performed as an emergency operation to successfully decompress the colon.
The goals of palliation are different from those of curative resection and include avoidance of complications, a shorter hospital length of stay, fewer healthcare transitions, and appropriate referrals to hospice [4, 6, 9-11, 13, 14, 17]. Therefore, beyond the clear benefits of blowhole colostomy, such as reduced likelihood of prolonged hospital stay, other benefits (such as reduced likelihood of prolonged operative time and surgical complications) are of particular importance in palliative management of complete colonic obstruction.
This study had several limitations. Above all, in our institution, the decision of which surgical procedure to choose for acute complete colonic obstruction depended on the operator’s preference. Therefore, the indications for blowhole colostomy and loop ostomy were different, which is a significant limitation of this study. In order to supplement this in the future, more specific indications should be determined, and additional research should be conducted. The inclusion criteria were not controlled strictly because the data were not collected prospectively. Retrospective case-control designs have some inherent difficulties, such as assessing causality and the risk of selection bias. The number of patients who could be evaluated adequately for ostomy-related complications was limited. Future studies will need to further investigate prospective evaluation of blowhole colostomy versus loop ostomy for emergent decompression of terminal cancer patients. Diverting blowhole colostomy can be a safe and effective procedure with promisingly low morbidity and significant reduction in postoperative length of stay in emergency situations such as massive colonic distension. When a surgeon decides to create an ostomy, the blowhole type should not be considered inferior to the loop type, at least for colonic decompression.
Fig. 1.
(A) Photograph of a blowhole colostomy performed on the cecum. (B) Photograph of a blowhole colostomy performed on the transverse colon. This patient was operated under local anesthesia because general anesthesia was impossible due to very severe chronic obstructive pulmonary disease. (C) Photograph of a spontaneously closed blowhole colostomy on the transverse colon.

Fig. 2.
(A) Abdominal X-ray before surgical intervention. (B) Abdominal X-ray after blowhole colostomy on the transverse colon at postoperative day 1.

Table 1.
Patient characteristics by loop ostomy and blowhole colostomy
| Characteristic | Total | Loop type | Blowhole type | P-value |
|---|---|---|---|---|
| No. of patients | 59 | 35 | 24 | |
| Age (yr) | 68.83 ± 12.45 | 68.46 ± 12.32 | 69.38 ± 12.88 | 0.784 |
| Sex | 0.821 | |||
| Female | 26 (44.1) | 15 (42.9) | 11 (45.8) | |
| Male | 33 (55.9) | 20 (57.1) | 13 (54.2) | |
| ASA PS classification | III (II–III) | III (II–III) | III (II–III) | 0.481 |
| Albumin (g/dL) | 3.37 ± 0.65 | 3.38 ± 0.67 | 3.36 ± 0.63 | 0.914 |
| Body mass index (kg/m2) | 20.56 ± 3.73 | 21.06 ± 3.84 | 19.84 ± 3.51 | 0.22 |
| Cecum diameter (cm) | 7.69 ± 2.38 | 6.78 ± 2.36 | 8.83 ± 1.91 | 0.001 |
| Ostomy site | < 0.001 | |||
| Ileum | 16 (27.1) | 16 (45.7) | 0 (0) | |
| Cecum | 13 (22.0) | 0 (0) | 13 (54.2) | |
| Transverse colon | 17 (28.8) | 7 (20.0) | 11 (45.8) | |
| Sigmoid colon | 13 (22.0) | 12 (34.3) | 0 (0) | |
| Timing of the surgery | 0.021 | |||
| Elective | 14 (23.7) | 12 (34.3) | 2 (8.3) | |
| Emergencya | 45 (76.3) | 23 (65.7) | 22 (91.7) |
Table 2.
Surgical outcomes in loop ostomy and blowhole colostomy
Table 3.
Comparison of the postoperative complications of loop ostomy and blowhole colostomy according to Clavien-Dindo (CD) classification grade
REFERENCES
1. Kasten KR, Midura EF, Davis BR, Rafferty JF, Paquette IM. Blowhole colostomy for the urgent management of distal large bowel obstruction. J Surg Res 2014;188:53–7.


2. Kuk JC, Jung EJ, Ryu CG, Moon SM, Hwang DY. Usefulness of an open cecostomy in the treatment of a distal colon obstruction. J Korean Soc Coloproctol 2010;26:111–5.

3. Amelung FJ, Mulder CL, Broeders IA, Consten EC, Draaisma WA. Efficacy of loop colostomy construction for acute left-sided colonic obstructions: a cohort analysis. Int J Colorectal Dis 2017;32:383–90.


4. Nagashima Y, Funahashi K, Ushigome M, Kagami S, Kaneko T, Yoshino Y, et al. Comparative outcomes between palliative ileostomy and colostomy in patients with malignant large bowel obstruction. J Anus Rectum Colon 2019;3:73–7.



5. Krstic S, Resanovic V, Alempijevic T, Resanovic A, Sijacki A, Djukic V, et al. Hartmann’s procedure vs loop colostomy in the treatment of obstructive rectosigmoid cancer. World J Emerg Surg 2014;9:52.



6. Ribeiro IB, Bernardo WM, Martins BD, de Moura DT, Baba ER, Josino IR, et al. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open 2018;6:E558–67.



7. Targownik LE, Spiegel BM, Sack J, Hines OJ, Dulai GS, Gralnek IM, et al. Colonic stent vs. emergency surgery for management of acute left-sided malignant colonic obstruction: a decision analysis. Gastrointest Endosc 2004;60:865–74.


8. van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 2011;12:344–52.


9. Tomiki Y, Watanabe T, Ishibiki Y, Tanaka M, Suda S, Yamamoto T, et al. Comparison of stent placement and colostomy as palliative treatment for inoperable malignant colorectal obstruction. Surg Endosc 2004;18:1572–7.


10. Abelson JS, Yeo HL, Mao J, Milsom JW, Sedrakyan A. Long-term postprocedural outcomes of palliative emergency stenting vs stoma in malignant large-bowel obstruction. JAMA Surg 2017;152:429–35.



11. Fiori E, Lamazza A, Schillaci A, Femia S, Demasi E, Decesare A, et al. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg 2012;204:321–6.


12. Ripamonti C, Twycross R, Baines M, Bozzetti F, Capri S, De Conno F, et al. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001;9:223–33.


13. Pickard C, Thomas R, Robertson I, Macdonald A. Ostomy creation for palliative care of patients with nonresectable colorectal cancer and bowel obstruction. J Wound Ostomy Continence Nurs 2018;45:239–41.


14. Zhao XD, Cai BB, Cao RS, Shi RH. Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol 2013;19:5565–74.



15. Jaffe T, Thompson WM. Large-bowel obstruction in the adult: classic radiographic and CT findings, etiology, and mimics. Radiology 2015;275:651–63.


16. Jochum SB, Bhama AR. Radiographic guidance for TurnbullWeakley blowhole colostomy. Tech Coloproctol 2021;25:349–50.


17. Mäkelä J, Haukipuro K, Laitinen S, Kairaluoma MI. Palliative operations for colorectal cancer. Dis Colon Rectum 1990;33:846–50.


18. Engida A, Ayelign T, Mahteme B, Aida T, Abreham B. Types and indications of colostomy and determinants of outcomes of patients after surgery. Ethiop J Health Sci 2016;26:117–20.



20. Correa-Marinez A, Grenabo J, Bock D, Wedin A, Angenete E. The type of stoma matters-morbidity in patients with obstructing colorectal cancer. Int J Colorectal Dis 2018;33:1773–80.


21. Tsujinaka S, Tan KY, Miyakura Y, Fukano R, Oshima M, Konishi F, et al. Current management of intestinal stomas and their complications. J Anus Rectum Colon 2020;4:25–33.



22. Fontes B, Fontes W, Utiyama EM, Birolini D. The efficacy of loop colostomy for complete fecal diversion. Dis Colon Rectum 1988;31:298–302.


24. Yeom SS, Kim CW, Jung SW, Oh SH, Lee JL, Yoon YS, et al. Trephine transverse colostomy is effective for patients who have previously undergone rectal surgery. Ann Coloproctol 2018;34:72–7.



25. Remzi FH, Oncel M, Hull TL, Strong SA, Lavery IC, Fazio VW. Current indications for blow-hole colostomy:ileostomy procedure: a single center experience. Int J Colorectal Dis 2003;18:361–4.


26. Youssef F, Arbash G, Puligandla PS, Baird RJ. Loop versus divided colostomy for the management of anorectal malformations: a systematic review and meta-analysis. J Pediatr Surg 2017;52:783–90.


27. Kerstens J, Diebels I, de Gheldere C, Vanclooster P. Blowhole colostomy for Clostridium difficile-associated toxic megacolon. Case Rep Surg 2016;2016:5909248.




28. Ooi BS, Remzi FH, Fazio VW. Turnbull-Blowhole colostomy for toxic ulcerative colitis in pregnancy: report of two cases. Dis Colon Rectum 2003;46:111–5.

-
METRICS

- Related articles in ACP
-
Usefulness of an Open Cecostomy in the Treatment of a Distal Colon Obstruction.2010 April;26(2)