- Search
| Ann Coloproctol > Volume 39(3); 2023 > Article |
|
Abstract
Purpose
The anatomical distribution of perianal warts is associated with patient characteristics such as sexual orientation. The purpose of this study is to confirm this experiential knowledge using a quantitative classification system and analysis and to obtain findings useful for future treatment.
Methods
From January 2014 to December 2020, 93 patients with perianal warts presented to our hospital. Patients were analyzed for age, sex, lesion site, and recurrence type, among other factors. The lesion site was divided into skin (S) and anal epithelium (anoderm, A), and the number and degree of each were classified into grades 0 to 3. The higher grade between S and A determines its dominant type, such as type S (e.g., S3A1) and type A (e.g., S0A2).
Results
The average age of the patients was 39.6 years, and the percentage of patients who were not married was 54.8%. In all, 95.8% of patients were positive for low-risk human papillomavirus (HPV). Type S accounted for 80.6%, whereas type A accounted for 9.7%. Type A cases were all male and were all presumed to be men who have sex with men (MSM). This indicates that the determination of type A may be highly specific for MSM. The type at the time of recurrence was the same type at the time of the first surgery in almost all cases.
Anogenital warts result from local infections with human papillomavirus (HPV), which is one of the most common sexually transmitted infections (STIs). According to the “2015 Sexually transmitted diseases treatment guidelines” published by the CDC [1], approximately 100 types of HPVs have been identified, at least 40 of which can infect the genital area. Most sexually active individuals become infected with HPV at least once in their lifetimes. Oncogenic, high-risk HPV infection (e.g., HPV types 16 and 18) causes most cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancers and precancerous lesions, whereas nononcogenic, low-risk HPV infection (e.g., HPV types 6 and 11) causes genital warts and recurrent respiratory papillomatosis [1]. Substantial proportions of cancers and anogenital warts are attributable to HPV in the United States. In 2009, an estimated total of 34,788 new HPV-associated cancers and approximately 355,000 new cases of anogenital warts were associated with HPV infection.
In women, anogenital warts can appear on the vulva, vaginal walls, the area between the external genitals and the anus, the anal canal, and the cervix. In men, they may occur on the penis, scrotum, and anus [1]. Because we are practicing proctologists, most of our patients with anogenital warts present with only perianal warts. In this report, the term “perianal” refers to skin around anus and anal canal (anoderm). Many reports on perianal warts have been published, some of which discuss treatment methods and recurrence risk factors from analyses based on shapes, such as comb, chicken-crown, and cauliflower-like shapes.
In daily practice, proctologists experientially know that perianal warts are associated with different patient characteristics and recurrence rates depending on the shape of warts and depending on whether the site of occurrence is the skin around the anus or the anal epithelium (anoderm in the anal canal). However, no report has quantitatively classified perianal warts, and no report has confirmed such experiential knowledge. In this study, by grading and analyzing the degree and site of perianal warts, we report findings that are useful for daily clinical practice. Especially it is useful for predicting coinfections and hidden sex orientations for the first time we encounter the perianal warts patient.
This is a retrospective cohort study in which medical records were analyzed. This study protocol was reviewed and approved by the Ethical Committee of Tokatsu-Tsujinaka Hospital (No. 2020CS-6). Informed consent was waived due to the retrospective nature of the study.
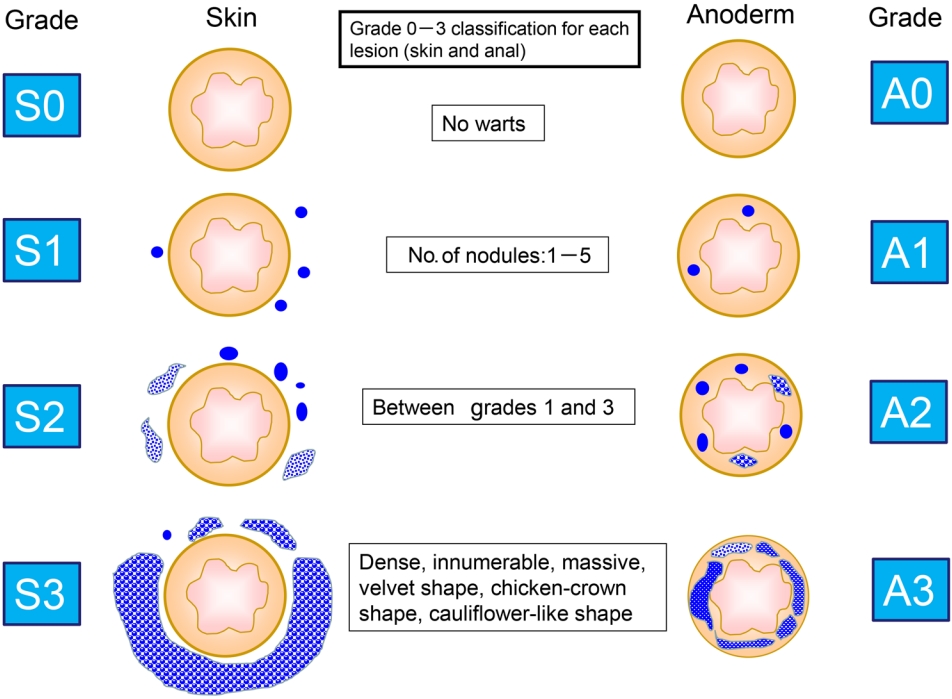
From January 2014 to December 2020, we treated 93 cases of perianal warts at our hospital. Patients whose records only included posttreatment follow-up were excluded. We made a new classification based on the extent and distribution of perianal warts. The numbers of skin (S) and anal epithelium (anoderm, A) cases were classified into grades 0 to 3 based on the number of nodules that were visible on 1 or both sides: grade 0, no warts; grade 1, 1 to 5; grade 2, between grades 1 and 3; grade 3, warts that were dense, innumerable, and massive with a velvet shape, chicken-crown shape, or cauliflower-like shape (Fig. 1). The distribution of the lesions and the classification of the number of lesions were based on sketches in the electronic medical records. At our hospital, all electronic medical records are the touch-pen type, and all records contain sketches of anal findings from the outpatient examination. All surgical records are also illustrated with a stylus. In this study, sketches of anal findings and surgical records were referenced. No cases showed a difference in classification between outpatient examination findings and surgical records. Cases for which the records include a description such as “2 on the skin” or “several on the anal epithelium” were classified as S1 and A1, respectively. In cases without descriptions, the points of warts on the sketch were counted, and the type of wart was determined from the sketch. Some records contained descriptions, such as “dense,” “giant,” “cauliflower-like growth,” and “chicken-crown-like,” which were classified as grade 3. Between S and A, the wart with a higher grade decided the type for that case, and warts with the same grade between S and A were classified as the equivalent type. For example, S3A1 is type S (skin-dominant type), S0A2 is type A (anal-dominant type), and S1A1 is the equivalent type.
To assess HPV infection, we used a nucleic acid hybridization method in which a swab was applied to scrape the perianal skin.
The average postoperative follow-up period was 30.3 months. We checked them in the outpatient room on the 3rd, 10th, and 1st months after surgery. But some patients did not appear on the appointment day. After that, they were checked once every 1 to 2 months until 6 months after the surgery, and then in the 1st year. The postoperative recurrence time was counted by month. If it is less than 15 days, it will be rounded down, and if it is 15 days or more, it will be rounded up. For example, recurrence on the 10th day was counted as recurrence in 0 month, and recurrence on the 50th day was counted as recurrence in 2 months.
The chi-square test was used to test for significance. We used IBM SPSS ver. 28.0 (IBM Corp) for statistical analysis. Statistical significance was defined as a P-value of < 0.05.
The average age of the 93 patients was 39.6 years; and of these, 62 were male and 31 were female (Table 1). The percentage of unmarried patients was 54.8% in each male and female. Of the 93 cases, 7 male patients, including 6 who were homosexual and 1 who was bisexual, acknowledged during the interview that they had experienced homosexual intercourse (Table 2).
Of the 93 cases, 78 were diagnosed for the first time (first diagnosis at our hospital or introduction from another hospital), 7 patients had a history of perianal warts at our hospital, and 8 patients had a history of perianal warts at other hospitals. The diagnostic rate by inspection at the first visit was 100%. The final pathologic confirmation of perianal warts was 90 of 93 (96.8%). Pathologic examinations were not performed for the remaining 3 cases because of conservative treatment (Table 1).
HIV infection was assessed in 74 of 93 cases. Four male patients (5.4%) were HIV-positive, all of whom were male. Two of the 4 patients were under treatment, and the other 2 were newly diagnosed. Syphilis was assessed in 80 cases; 6 cases (7.5%, all male) were positive for syphilis. Five of 6 cases had undergone previous treatment for syphilis, and 1 case was newly diagnosed. Of these 6 syphilis cases, 3 were coinfected with HIV (Table 2).
Examinations for HPV infection were performed using the nucleic acid hybridization method. Because this test was performed at each patient’s expense, only 48 of 93 patients agreed to this test (Table 2). Of those 48 patients, 34 (70.8%) were positive for only low-risk HPV, and 12 cases (25.0%) were positive for both low -and high-risk HPV. No patients were positive for only high-risk HPV, and 2 cases (4.2%) were negative for both low- and high-risk HPV. The patients positive for low-risk HPV accounted for 95.8% of all patients who agreed to the test. Of the 12 cases that were positive for both low- and high-risk HPV; 10 were male and 2 were female. Of these 10 male cases, 4 were homosexual and 1 was bisexual.
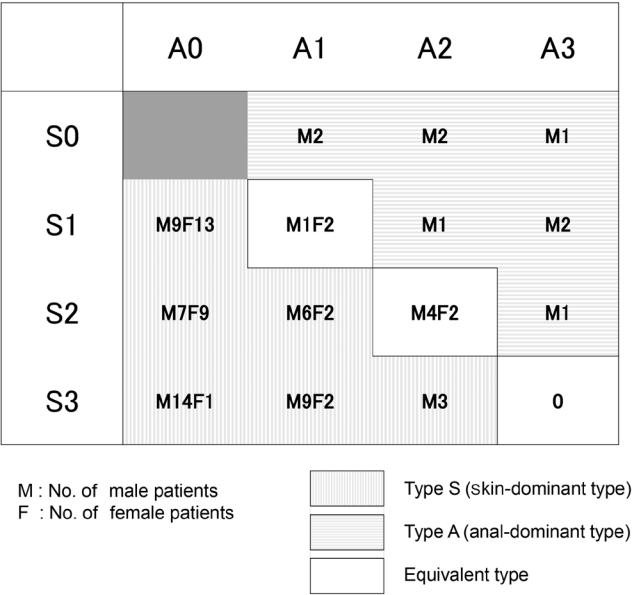
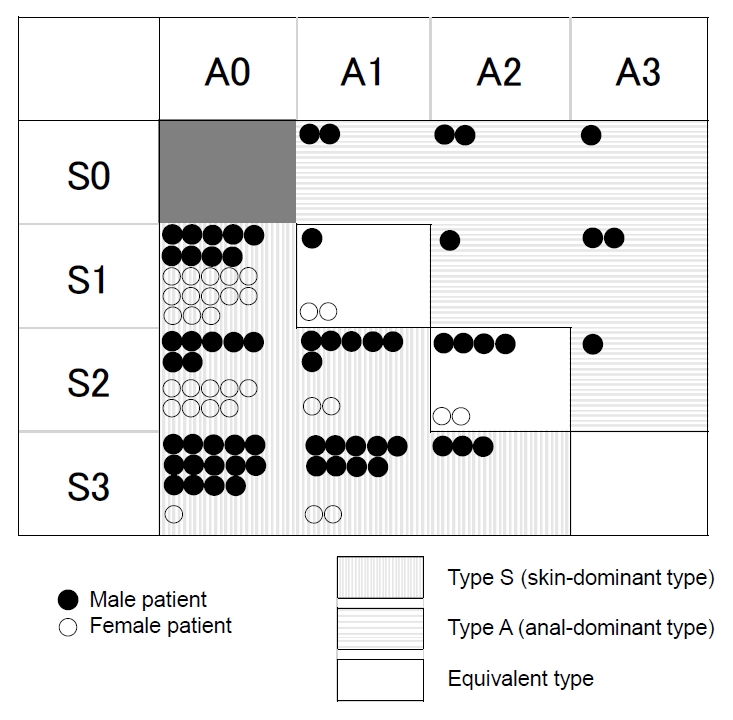
Regarding the classification, type S was the most common, with 80.6% overall; 77.4% of male and 87.1% of female patients (Table 2). Fig. 2 shows the details of all 93 patients. The characteristics of all patients were plotted in a figure for enhanced visual understanding (Fig. 3).
Nine cases (all male) were classified as type A. The average age of type A patients was 28.8 years, and 100% were unmarried. Five of the 9 type A patients (all male) were positive for syphilis and 2 of 5 were also positive for HIV. On the other hand, 5 of the 9 type A patients accepted HPV infection test. Four of whom (80.0%) were positive for both low- and high-risk HPV, and 1 case (20.0%) was positive for only low-risk HPV.
This rate for both low- and high-risk HPV positivity in type A patients (80.0%) was higher than the overall rate for both low- and high-risk HPV positivity (25.0%). Six of these 9 male patients disclosed their homosexuality during the interview. The other 3 denied being homosexual. However, 2 of 3 patients who denied being homosexual were coinfected with syphilis and HIV, and the third patient was infected with syphilis only.
Of the 93 cases, 6 did not undergo surgery, and 87 cases were treated by resection. Of the 6 patients who did not undergo surgery, 4 requested conservative treatment with Bethelna ointment, 1 patient canceled the surgery, and the remaining patient was referred to an infectious disease core hospital because of syphilis and HIV coinfection.
Of the 87 resected cases, 20 (23.0%) were performed under local anesthesia, 66 (75.9%) were performed under lumbar anesthesia or saddle block, and 1 (1.1%) was performed under general anesthesia (Table 1). The patient who received general anesthesia was a 1-year-old girl. Throughout the course, 19 patients (20.4%) used Bethelna ointment. Of these 87 surgical cases, 35 (40.2%) experienced recurrence during the follow-up period (Table 3). The average postoperative period at which recurrence was confirmed was 3.4 months, the median period was 2 months, and the mode was 1 month (Fig. 4). Recurrence within 6 months occurred in 30 of 35 patients (85.7%), and recurrence within 1 year occurred in 34 of 35 patients (97.1%). The average overall number of surgeries was 2.2 in all 87 surgical cases and 3.9 in the 35 recurrent cases. Table 3 shows the analysis of each type for cases with and without recurrence. In nonrecurrent patients, preoperative type A accounted for 5.8%. In those who relapsed after surgery, preoperative type A accounted for 14.3%. The ratio of preoperative type A was higher in the postoperative recurrence group. However, no statistically significant difference was observed in the distribution of the preoperative type, between the nonrecurrent and recurrent groups, and the P-value of the chi-square test was 0.157.
At the time of postoperative recurrence in 35 patients, the S and A grades fluctuated only slightly, but almost all cases were the same type at first time and second time surgery (Table 4). The type changed in only 1 case, from type A at the time of initial surgery to the equivalent type at the time of recurrence, because the lesion in the anal canal was largely healed. The skin only type (S1–3A0) and the anal epithelium only type (S0A1–3) were both A0 and S0 at the time of recurrence. No new lesions appeared in any case at the time of recurrence.
Toyonaga et al. [2] reported that the average patient age was 37.7 years in a study of 122 cases of perianal warts. The peak ages for male and female patients were their 30s and 20s, respectively. At our hospital, the median age was the late 30s for both male and female patients. In addition, by analyzing “the disease outbreak trend survey 2004 from the Ministry of Health, Labour, and Welfare”, Matsuda [3] reported that males peaked in their 30s and had dome-shaped wart outbreaks in their 20s and 40s. By contrast, females peaked in their 20s, whereas the incidence of warts decreased in their 30s. According to these data, patients with perianal warts in Japan are, on average, in their late 30s, and the mode and median age are the 30s for males and the late 20s to early 30s for females.
According to the demographic data of 2019 from the National Institute of Population and Social Security Research [4], the average ages at first marriage are 31.1 years for men and 29.4 years for women. According to the percentage of unmarried patients every 5 years from the same report, the percentage of unmarried men aged 35 to 39 years is 35.0%, that of men aged 40 to 44 years is 30.0%, and the percentage of unmarried women aged 35 to 39 years is 23.9%. This suggests that the value of 54.8% of all patients with perianal warts at our hospital (average age, 39.6 years) is high. Toyonaga et al. [2] also reported that the percentage of unmarried patients was 55.7% of 122 patients (average age, 37.7 years).
It is difficult to determine the relationship between a high number of unmarried patients and the occurrence of perianal warts because many other factors are involved. However, given that perianal warts are caused by an STI, it is likely that the high number of unmarried patients is related to sexual activity, sexual diversification, and sexual contact with multiple partners.
In our patients, only 4.2% were negative for low-risk HPV. Considering that 95.8% were positive for low-risk HPV, the sensitivity of the nucleic acid hybridization method is acceptable. In cases negative for low-risk HPV, other skin diseases, such as verruca vulgaris, should also be considered.
We termed the new classification the “SA classification.” As many surgeons have realized, all 9 cases classified as type A (analdominant type) in the SA classification were male. Six of the 9 type A male patients acknowledged their homosexuality during the interview. The remaining 3 who denied being homosexual were infected with both syphilis and HIV or syphilis alone. According to the “2016 AIDS trends annual report of the Ministry of Health, Labour, and Welfare” [5], there were 1,011 cases of HIV infection in Japan in 2016, of which 857 were Japanese men, and 78.1% of male patients were infected during same-sex intercourse. According to data from the National Institute of Infectious Diseases [6], 73.4% of the 10,997 domestic syphilis patients from 2012 to 2016 were male, and on the basis of self-reporting, 49.0% of them were infected during heterosexual contact, 36.3% were infected during same-sex contact, and 13.6% were infected through an unknown cause. According to a report [7] from the proctology department of a general hospital that is also a core AIDS hospital, of 8,609 patients who underwent preoperative examination, 38 were HIV-positive. All of those patients were male, and 2/3 of them reported same-sex anal intercourse. The report states that 30% of patients did not respond to the question regarding sexual contact. The report also states that there is a limit to determining the truth regarding sexual orientation by self-reporting because some believe that questions on private sexual behavior are distasteful.
In our study, all 3 male patients with type A who denied being men who have sex with men (MSM) were found to have syphilis or syphilis+HIV superinfection. Considering the above data, it is reasonable to assume that these 3 males were actually MSM. On the basis of this assumption, all 9 type A patients are MSM. In other words, the type A classification may have 100% specificity for MSM. Consent for HPV testing was obtained in 5 of these 9 cases of type A male patients. In these 5 cases, 1 case (20.0%) was positive for only low-risk HPV, and 4 cases (80.0%) were positive for both low- and high-risk HPV. In comparison, the positivity rate for both low- and high-risk HPV in all 48 patients who agreed to test was 25.0%. If we encounter patients with type A perianal warts, at the first visit, we should consider the possibility of infection with syphilis, HIV, and both low- and high-risk HPV, as well as all other STIs. Because anal intercourse can be accompanied by mucosal damage, patients who have anal sex are at a higher risk for STI than those who have vaginal intercourse, and preoperative examinations for such infections are essential before surgery.
Ong et al. [8] reported on 15,590 MSM patients at the Melbourne Sexual Health Center from 2016 to 2018. In all, 9,576 men reported receptive anal sex, 9,846 reported insertive anal sex, and 10,762 reported both receptive and insertive anal sex. Overall, 432 of 9,576 men (4.5%) who reported receptive anal sex had anal warts, 143 of 9,846 men (1.5%) who reported insertive anal sex had penile warts, and 14 of 10,762 men (0.1%) who reported both receptive and insertive anal sex had both anal and penile warts. A clear difference was observed in perianal wart lesions due to the sex type. In the same report, anal warts were most frequent in younger MSM (5.8% for those aged < 21 years) and were less frequent with increasing age (2.8% in those aged > 50 years). The authors suggest some reasons for this; one reason is that susceptibility to HPV differs according to age. Specifically, anal HPV is acquired more easily in younger MSM and may indicate that the anal epithelium of younger MSM may be more susceptible to HPV infection than that of older MSM. In our cases, the 9 type A male patients were 23 to 38 years of age, and the average age was 28.8 years. Penile warts were not observed in our cases. Judging from the above report, the sex type in our male patients was assumed to be receptive anal sex.
Galea et al. [9] reported on 600 Peruvian MSM and trans women. They reported that HPV infection specifically in the anal canal among MSM occurs rapidly after sexual intercourse and persists in approximately 60% of individuals throughout their lives. They also state that some risk factors, such as the number of recent male sex partners and both recent and lifetime histories of receptive anal intercourse, are shared risk factors for HPV and HIV infection. According to their study, owing to the discomfort posed by sex-related questions during the interview, some patients do not readily acknowledge that they are MSM. In those cases, visible anogenital warts are useful for advancing medical treatment. Owing to the intimate nature of questions on sexual behaviors and STI risks, the collection of detailed and accurate data used to evaluate HIV risk is challenging. Moreover, a visible marker of HIV risk, such as visible anogenital warts, would be especially useful for clinicians treating MSM who may be reluctant to disclose same-sex behaviors.
Toyonaga et al. [2] reported that 48% of patients experienced recurrence after resection, and recurrence occurred within 1 month after surgery in 46% and within 6 months in 99%. In a subsequent report, Okamoto et al. [10] reported a 92% of recurrence rate within a year. According to our data, 85.7% of patients experienced recurrence within 6 months, and 97.1% experienced recurrence within 1 year. On the basis of these data, it is necessary to follow up with patients after surgery for at least 6 months but preferably for 1 year.
Regarding postoperative recurrence, almost all cases of recurrence have the same type as before surgery. Specifically, in recurrent patients with skin only (A0) or anal epithelium only (S0) lesions, 100% of patients with recurrence also have A0 and S0, and no case was observed in which the lesion spread across S and A in the individual at the time of recurrence. These results indicate that it is highly possible that the lesion range is defined by first contact during the initial infection. This is what proctologists have experientially assumed, and this has finally been confirmed by the SA classification. In cases where the lesion spreads across S and A at the time of recurrence, iatrogenic spread, such as by intraoperative scraping in a previous surgery, should be considered.
We think the classification system we developed is useful in daily clinical practice for predicting coinfections, such as syphilis and HIV, and also predicting the hidden sex orientations for the first time we encounter the perianal warts patient, especially with type A perianal warts.
Notes
AUTHOR CONTRIBUITONS
Conceptualization: all authors; Data curation: JU; Formal analysis: JU; Investigation: all authors; Methodology: JU, KM; Project administration: KM; Resources: KM; Software: JU; Supervision: KM; Validation: JU, KM; Visualization: JU; Writing–original draft: JU; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 4.
The time when recurrence was confirmed in 35 patients. The average was 3.4 ± 4.6 months and the median was 2 months.
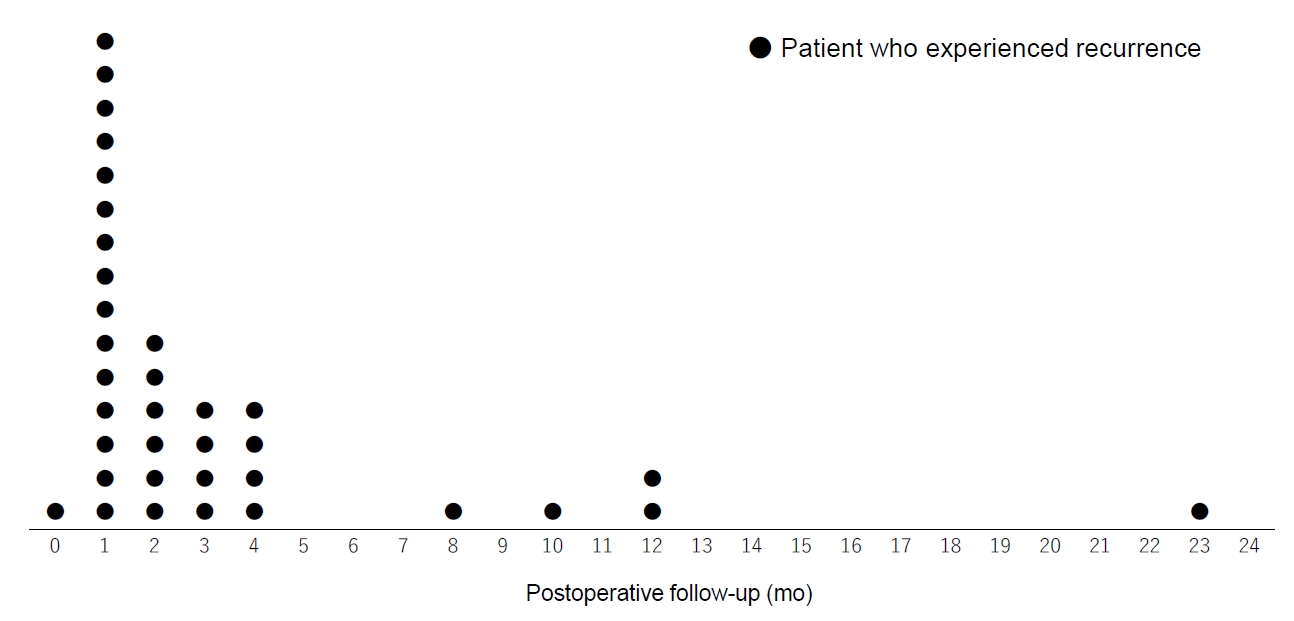
Table 1.
Demographics and clinical data of all 93 patients
Table 2.
Clinical data of each total, male, and female patients
Table 3.
Type analysis of nonrecurrent and recurrent cases
REFERENCES
1. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64(RR-03): 1–137.
2. Toyonaga T, Matsushima M, Katori R, Takahashi T, Kiryu K, Sogawa N, et al. Factors affecting recurrence after surgical excision for perianal warts. J Jpn Soc Coloproctol 2006;59:259–64.

3. Matsuda Y. A special edition-Sexually transmitted diseases of the anus and rectum. J Jpn Soc Coloproctol 2006;59:846–50.

4. National Institute of Population and Social Security Research. Demographic data (2019) [Internet]. National Institute of Population and Social Security Research; c2010 [cited 2021 Apr 20]. Available from: http://www.ipss.go.jp/syoushika/tohkei/Popular/Popular2019.asp?chap=0
5. AIDS Trend Committee; Ministry of Health; Labour and Welfare. AIDS occurrence trends annual report 2016 [Internet]. Ministry of Health, Labour and Welfare of Japan; 2016 [cited 2021 Apr 20]. Available from: https://api-net.jfap.or.jp/status/japan/data/2016/nenpo/h28gaiyo.pdf
6. Takahashi T, Arima Y, Yamagishi T, Nishiki S, Kanai M, Ishikane M, et al. Rapid increase in reports of syphilis associated with men who have sex with women and women who have sex with men, Japan, 2012 to 2016. Sex Transm Dis 2018;45:139–43.



7. Yamana T. The prevalence of HIV infection in preoperative patients with benign anal diseases. J Jpn Soc Coloproctol 2006;59:827–31.

8. Ong JJ, Fairley CK, Read TR, Chen MY, Zhang L, Chow EP. Age-specific pattern of anogenital wart in men who have sex with men. Sex Transm Dis 2018;45:186–8.


- TOOLS