- Search
| Ann Coloproctol > Volume 40(2); 2024 > Article |
|
Abstract
Purpose
Surgeons can treat debilitating conditions of uncontrollable complex anorectal fistulas with sepsis, even after repeated fistula surgeries, for curative intention. Adipose-derived stem cells have shown good outcomes for refractory Crohn fistula. Unfortunately, cell therapy has some limitations, including high costs. We have therefore attempted immediate cell-assisted lipotransfer (CAL) in treating refractory complex anal fistulas and observed its outcomes.
Methods
In a retrospective study, CAL, using a mixture of freshly extracted autologous stromal vascular fraction and fat tissues, was used to treat 22 patients of refractory complex anal fistula from March 2018 to May 2021. Preoperative and postoperative assessments were performed with direct visual inspection, digital palpation, and endoanal ultrasonography. A fistula was considered completely healed if (1) the patient had no symptoms of discharge or inflammation; (2) there were no visible secondary openings of fistula tract inside and outside of the anorectal unit and even in the perineum; and (3) there was no primary opening in the anus. The endpoint of complete remission was wound healing without signs of inflammation 3 months after CAL treatment.
Results
In a total of 22 patients who received CAL treatment, 19 patients showed complete remission, 1 patient showed partial improvement, and 2 patients showed no improvement. One of the 2 patients without improvement at primary endpoint showed complete remission 9 months after CAL. There were no significant adverse effects of the procedure.
Conclusion
We found that the immediately-collected CAL procedure for refractory complex anal fistula showed good outcomes without adverse side effects. It can be strongly recommended as an alternative surgical option for the treatment of complex anal fistula that is uncontrollable even after repeated surgical procedures. However, considering the unpredictable characteristics of stromal vascular fraction, long-term follow-up is necessary.
Surgical treatment of complex anal fistula remains challenging, with a high risk of complications such as infection, delayed wound healing, recurrence, fecal incontinence (FI), and even uncontrollable inflammation. The goals of the treatment of anal fistula include resolving the acute and chronic inflammatory process, maintaining continence, and preventing future recurrence [1]. Through long-term efforts in care and research, surgeons have developed familiarity with the anatomy of the anorectal unit and with the pathophysiology of anal fistula. Due to the development and improvement of sophisticated operative technologies, as well as creative, advanced surgical techniques, we have achieved substantial results in the treatment of anal fistula. Although we actively pursue our work based on sound surgical principles, we colorectal surgeons must still deal with relapses and complex anal fistula cases that do not heal. These cases can be categorized as refractory complex anal fistula.
Treatment of perianal fistulas with cultured mesenchymal stem cells derived from adipose tissue or bone marrow has shown promising results in both patients with [2, 3] and without Crohn disease [4]. Furthermore, the efficacy and safety of adipose-derived stem cell (ADSC) treatment in refractory complex anal fistulas of cryptoglandular origin are well known [2, 3, 5, 6]. There are also other studies of refractory anal fistula treatments that use autologous fat tissues [7] or microfragmented fats [6, 8]. However, there remain obstacles in terms of cost and time in ADSC treatment, and hospitals face challenges in providing proper facilities and equipment, as well as in securing the proper expertise and technical know-how.
Stromal vascular fraction (SVF) can be obtained through enzymatic and nonenzymatic isolation techniques. We isolated SVF through an enzymatic isolation process that mixed lipoaspirates with collagenase type I, and we used a closed isolation kit system. Through these kits, we were able to secure sufficient SVF for well-established on-site surgical procedures, with minimal equipment needed for centrifugation and washing, and without the need for major equipment and facilities or specialist researchers. We were able to use this SVF effectively by mixing it with newly-harvested fat tissues from the operating room. This is the treatment that we refer to here as cell-assisted lipotransfer (CAL).
The aim of this review is to investigate the efficacy of CAL in treating refractory complex anal fistula with inflammation that is unresponsive to any other known surgical procedures. We categorized as complex anal fistula those fistulas that could not be easily treated, as described in the discussion below.
This study was approved by the Public Institutional Review Board designated by the Korea National Institute for Bioethics Policy, with a waiver for informed consent (No. P01-202202-01-002).
We report treatment results obtained from the use of CAL from a freshly collected mixture of isolated SVF and autologous fat tissues, which were locally injected in 22 patients with otherwise untreatable refractory complex anal fistula from March 2018 to May 2021. Our primary endpoint was complete healing of anal fistula and surrounding inflammation 3 months after the CAL procedure. Inclusion criteria consisted of complex anal fistulas refractory to surgical procedures, including repeated recurrence even after radical excision of the anal fistula tract and closure for at least 6 months. We collected data retrospectively through medical record reviews. When the patients visited our hospital for the first time, perianal fistulas were classified using Parks classification [9]. All patients included in our study had undergone at least 1 or more operation of anal fistula in our hospital before CAL.
The primary endpoint was complete fistula healing, as observed in clinical examinations 3 months after CAL injection. Visual inspection, digital examination, anorectal manometry, and endoanal ultrasonography were undertaken to determine outcomes. Anorectal manometry and endoanal ultrasonography were performed in some but not all patients. For some patients, we did magnetic resonance imaging to identify possible inflammation hidden from the inspection and sonographic process.
A fistula was considered completely healed if (1) the patient had no symptoms of discharge or inflammation; (2) there was no visible secondary opening of fistula tract inside and outside of the anorectal unit and even in the perineum; and (3) there was no primary opening in the anus. The secondary endpoint is 24 months after CAL treatment. The authors will follow up and report later.
Autologous fat tissues were harvested from a patient’s abdomen, flank, buttock, and inner or outer thighs, depending on the condition of the patient. In a patient with abundant fat tissues, fat harvesting was carried out from the abdomen. However, in thinner patients from whom it was difficult to harvest enough fats to proceed, fat tissues were taken from other parts of the body.
Extraction of SVF was undertaken using a closed system kit with an enzymatic isolation process. Usually, 50 mL of fats mixed with oil, tumescent, and plasma fluid were mixed with type I collagenase at a ratio of 1:1. The syringes containing the enzyme and fat tissues were warmed and shaken to react for 30 minutes in a 37 ºC incubator. The syringes were connected to the distribution tube of the component isolator (Smart X Kit, DongKoo Bio& Pharma). After inserting the fat tissue into the component isolator, centrifugation was performed at 1,500×g for 3 minutes. The aim of this procedure was to remove most of the fat layer and supernatant by moving the plunger to the upper levels, and to gain only a part of the bottom layer, which contained SVF. After undertaking this washing process 3 times, the SVF remaining at the bottom layer was collected (Fig. 1A). Using a filter, only pure SVF was collected into the syringe. Additional fat to be used as scaffold was harvested from both thighs. After centrifugation (1,240×g, 3 minutes), foreign substances were removed and only pure adipose tissue was left. This was mixed with the previously made SVF with scaffold in a ratio of 1:10 (SVF:scaffold) using transfer. The syringe was then shaken slowly and gently. After sufficient mixing, a cannula (1.5 gauge) was connected to the syringe, and finally, the mix was injected into the lesion (Fig. 1B). When we checked internal opening of fistula, we closed it with a 3-0 Vicryl (Ethicon) suture. But we could not find internal openings in many cases because CAL patients had already undergone several fistula surgeries.
Analysis was done using IBM SPSS Statistics ver. 19.0 (IBM Corp). Characteristics of complete remission, partial improvement, and no improvement were assessed using Spearman rank correlation coefficient. Comparisons were performed at a significance level of 0.05.
A total of 22 patients with refractory complex anal fistula were treated with CAL. The age distribution was from 28 to 71 years, and the median age was 47.5 years old. Twenty patients (90.9%) were male and 2 (9.1%) were female. There were 5 patients wtih underlying diabetes mellitus and ulcerative colitis. All patients had undergone many surgeries before the CAL procedure, with the median number of surgical procedures at 4. The median period of prevalence was 13 months (6 to over 360 months). We used Parks classification [9] for anal fistula: 2 cases of type II (9.1%), 8 cases of type III (36.4%), and 12 cases of type IV (54.5%). In order to enhance our understanding of the characteristics of inflammation, we classified them as sinus (S), diffuse infiltrative (DI), and mixed (S + DI). There were 5 of S type (22.7%), 10 of DI type (45.5%), and 7 of mixed type (31.8%). The median amount of isolated SVF was 6 mL (range, 2–7 mL), and the median amount of scaffold was 23.5 mL (range, 10–32 mL). These general characteristics are listed in Table 1.
In the 22 patients who received CAL treatment, after 3 months 19 patients showed complete remission, 1 patient showed partial improvement, and 2 patients showed no improvement. In 19 patients, there were no visual inflammation signs, and the endoanal ultrasound showed no signs of acute or chronic inflammation (Fig. 2). In the 19 patients with complete remission, 2 patients had immediate aggravation of inflammation after CAL and showed increased discharge within a week. However, this inflammation soon showed improvement after conservative treatment. One patient with partial improvement and persistent scanty discharge, furthermore, became well and resumed normal activity without severe disability in his daily life. Demographic characteristics and outcomes are listed in Table 2.
The median follow-up time was 15.5 months (range, 3–36 months). Patient 13, who did not show improvement at the endpoint, showed complete remission 9 months after CAL. Of those who initially showed complete remission, patient 17 showed signs of inflammation, which had to be relieved, approximately 18 months following CAL treatment, but did not develop a meaningful abscess. That patient continues to be monitored.
Patient 21 showed partial improvement after 3 months. After 6 months that patient was confirmed to have gained complete remission. There were no other significant adverse effects to the procedure.
These 22 patients did not show significant statistical differences according to age, sex, underlying disease, Parks classification, inflammation type, SVF amounts at the time of the CAL treatment, and amounts of scaffold, but they did show significant differences in the number of previous surgeries (P=0.031) and the duration of fistula illness (P=0.047). There were not enough cases to apply a Rostick statistical analysis of the differences between patients with good outcomes and those without. In a Spearman relational analysis, meaningful differences in the number of previous surgeries and the duration of fistula illness were detected.
Treatment of complex anal fistula is very challenging, and there is no gold standard surgical procedure. According to Corman [10], complex fistulas are those other than intersphincteric and low transsphincteric fistulas. The implication is, obviously, that they are more difficult to treat than conventional fistulas and, in addition, are associated with an increased risk of recurrence as well as a greater likelihood of impairment of control.
Perianal fistula is difficult to treat when the fistula tract crosses sphincters substantially (involving greater than 30% of the external sphincter), is anterior in a female, is recurrent, has multiple tracts, or is associated with preexisting FI, chronic diarrhea, local irradiation, inflammatory bowel disease, or even malignancy [9, 11–13].
The word “problematic” has also been used. Frenkel et al. [14] proposed a simplified classification based on the level of the fistula: low, mid, and highly complex. He hypothesizes that such a system would be more useful for determining the appropriate treatment and for predicting long-term outcomes.
There are several possible procedures in the treatment of complex anal fistula. Surgical options depend on the patient’s condition and on whether it is simple or complicated. Several factors such as position, direction, and length of anal fistula tract, combined amounts of abscesses, and the number of openings (internal or external) are also important factors in determining surgical options. Also important is the severity of inflammation and depth of tract, whether or not it involves anorectal sphincter muscles, which anorectal structures (rectal wall or even pelvic floor muscles) are involved in, and so on. And surgical techniques can be influenced by the surgeon’s experience, preferences, and course and background of training, even facilities and equipment available in the hospital [10, 11].
Risks of anal fistula surgery are infection, recurrence, and bowel incontinence. Serious infections can occur after fistula surgery and may need to be treated in the hospital. Even after radical surgery for curative intention, the fistula can sometimes recur. Bowel incontinence is the biggest potential risk after fistula surgery. If the fistula is more complicated, the risk for incontinence is higher [1, 15–17].
Known surgical options for anal fistulas are fistulotomy, seton techniques, fistulectomy and repair (direct closure, advancement flap procedure, muscle filling technique, etc.), ligation of intersphincteric fistula tract (LIFT) procedure, endoscopic ablation video-assisted anal fistula treatment (VAAFT), laser surgery, fibrin glue, bioprosthetic plug, and others [10, 11, 18–21].
The risk factors for recurrence of an anal fistula can be categorized as follows: first, factors related to the fistula anatomy and other comorbidities; second, preoperative assessment factors of lack of identifying internal opening and of anorectal anatomic structure; third, intraoperative deficiencies such as surgeon’s failure in entire excision of sepsis and improper selection of technique leading to recurrence; and fourth, factors related to postoperative care in preventing complications [1, 15–17, 21–24].
With further division of the anal sphincter muscles during the second surgery, full-blown FI may manifest [17]. This is particularly the case in female and elderly patients, patients with weak anal sphincters, and patients with preoperative FI [24]. The age and sex of the patient did not influence postoperative continence, nor did the surgeon or surgical technique appear as a risk factor, although after excluding preoperative incontinence patients, fistulotomy was the technique that showed a higher risk of incontinence [17]. Preoperative incontinence was a risk factor for postoperative incontinence. Cutting seton may also have resulted in FI of 5.6% to 25.2% [17, 25].
It is essential to localize the internal opening of anal fistula in the beginning [23]. Representative surgical options to overcome recurrence are techniques such as fistulotomy and immediate sphincter repair after fistulectomy. However, these techniques for recurrent anal complex fistula can bring postoperative FI. Therefore, interest in sphincter-preserving techniques, such as LIFT, VAAFT, sealing with fibrin glue, porcine dermal collagen, and bioprosthetic plugs, is on the rise. However, with a few exceptions, the scientific evidence is low due to the lack of clinical trials and the large variation in the presentations.
A new method of treating refractory complex perianal fistulas is to use stem cells, such as direct injection of mesenchymal stem cells and systemic administration of stem cells combined with local injection, using ADSC, SVF, CAL, etc. CAL, which injects a mixture of SVF and purified autologous fat tissue, is such an ADSC-enriched lipofilling technique [2–7, 26]. Although the exact mechanism behind the therapeutic effect of SVF has yet to be clarified, we can speculate that certain characteristics of SVF may exert beneficial actions in the treatment of acute and chronic inflammation.
We have seen aspirated fat tissues containing high amounts of growth factors such as basic fibroblast growth factor (bFGF), insulin growth factor (IGF)-1, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF)-BB; and we can also get them from SVF [27, 28]. It is well known that growth factors and proteases such as matrix metalloproteinase-9 play a role in angiogenesis, cell survival, cell proliferation, and adipogenesis. SVF may play an important role of regulation in angiogenesis, cell proliferation, differentiation, metabolism, promotion of adipose stem cell migration, fibroblast proliferation, wound healing, and regeneration. VEGF is the most prominent angiogenic growth factor that exerts its effects synergistically with bFGF [27, 28].
Autologous fat grafting has been used in diverse clinical fields for a while. Autologous fat tissues have been shown as an effective filler in soft tissue augmentation. The main limitation of this procedure is the unpredictable resorption and volume loss of the fat graft [29, 30]. In order to compensate for this deficiency, recently an increasing amount of research has focused on the use of ADSC to enrich the fat graft. CAL is expected to be effective in treating post-fistulectomy tissue defects by using soft tissue space augmentation together with anti-inflammatory treatment, which thereby enhances wound healing and tissue regeneration [31–33].
In examining the therapeutic efficacy of SVF, we expect multipotent cellular components to affect tissue regeneration. SVF has not only the self-renewal potency of ADSC, but high levels of growth factors such as epidermal growth factor, VEGF, bFGF, keratinocyte growth factor, PDGF, hepatocyte growth factor, transforming growth factor-β, and brain-derived neurotrophic factor.
The reason behind the authors’ high proportion of patients with complex anal fistula patients is the following: According to our research, patients from not only the city of Busan but also surrounding cities come to our hospital based on recommendations, after having undergone multiple unsuccessful surgical treatments elsewhere. They include patients whose perianal sepsis has not been solved despite undergoing radical procedures, patients who develop sinus problems, or those who develop DI inflammation even without sinus or fistula tract infections. The present research is an intention to treat and takes as its study subjects those patients who have long suffered from inflammatory secretions despite many surgical procedures.
Out of the 22 patients who received CAL treatment, 19 were observed to have complete remission, and patient 17 had inflammation detected after 18 months. However, the inflammation was minor in severity, and while drainage was undertaken, abscesses did not develop and therefore no further treatment was pursued. Meanwhile, patient 13, who was originally observed as having not experienced any improvement, was confirmed as having a complete remission after approximately 1 year. There was no case of serious complications related to the procedure.
Therefore, there are limitation to demonstrate the effectiveness of treatment. There were insufficient follow-up periods in our study. The additional long-term study is indispensable to prove effect of CAL.
We found that our immediately-collected CAL procedure for refractory complex anal fistula showed good outcomes without adverse side effects. This procedure can be strongly recommended as an alternative surgical option for the treatment of complex anal fistulas that remain uncontrolled even after repeated other surgical procedures. CAL has been a safe and relatively easy procedure to carry out. However, considering the unpredictable characteristics of SVF, long-term follow-up is mandatory.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: ISJ, SHH; Data curation: ISJ, SHH; Formal analysis: ISJ; Investigation: all authors; Methodology: ISJ, SHH; Project administration: all authors; Resources: ISJ, HMY, HJ; Software: ISJ; Supervision: SHH; Validation: ISJ, SHH, HJ; Visualization: ISJ, SHH, HJ; Writing–original draft: all authors; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 1.
(A) Stromal vascular fraction was collected at the bottom (red part). (B) The autologous fat and stromal vascular fraction were injected into the lesion.
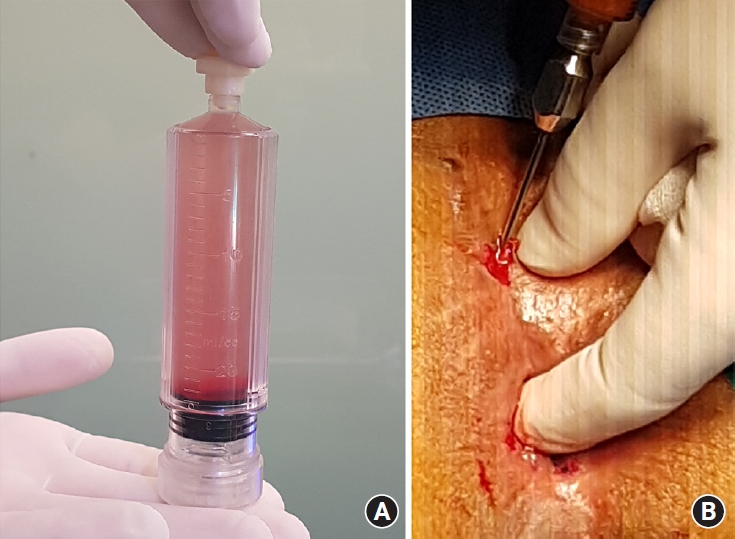
Fig. 2.
Diffuse infiltrative type with sinus in patient 20. Clincial images (A) before and (B) after cell-assisted lipotransfer.
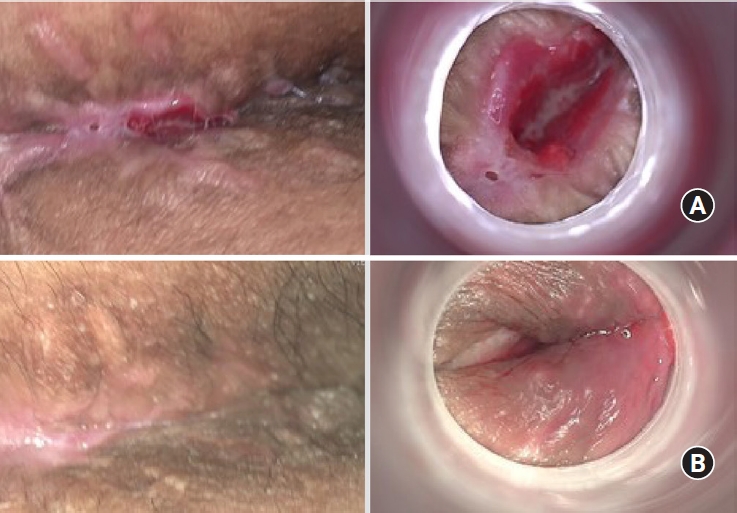
Table 1.
Characteristics of patients with fistula receiving cell-assisted lipotransfer (n=22)
| Characteristic | Value |
|---|---|
| Age (yr) | 47.5 (28–71) |
| Sex | |
| Female | 2 (9.1) |
| Male | 20 (90.9) |
| Underlying disease | |
| Diabetes mellitus | 4 (18.2) |
| Ulcerative colitis | 1 (4.5) |
| Previous operation | 4 (1–10) |
| Duration of fistula (mo) | 13 (6–360) |
| Parks classification [9] at the first visit to our hospital | |
| Type 1 | 0 (0) |
| Type 2 | 2 (9.1) |
| Type 3 | 8 (36.4) |
| Type 4 | 12 (54.5) |
| Inflammation | |
| Sinus | 5 (22.7) |
| Diffuse infiltrative | 10 (45.5) |
| Mixed | 7 (31.8) |
| Amount of stromal vascular fraction (mL) | 6 (2–7) |
| Amount of scaffold (mL) | 23.5 (10–32) |
| Improvement extent at 3 mo after surgery | |
| Complete remission | 19 (86.4) |
| Partial remission | 1 (4.5) |
| No improvement | 2 (9.1) |
| Follow-up (mo) | 15.5 (3–36) |
Table 2.
Clinical characteristics and outcome of individual patients
| Patient no. | Sex | Age (yr) | Underlying disease | No. of surgery | Duration of fistula (mo) | Parks classification [9] | Inflammation feature | SVF (mL) | Scaffold (mL) | Outcome at 3 mo after surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 64 | 7 | 360 | Type 4 | S+DI | 4.0 | 21.0 | No improvement | |
| 2 | Male | 65 | Diabetes mellitus | 6 | 9 | Type 4 | DI | 2.0 | 20.0 | Complete remission |
| 3 | Female | 51 | 4 | 14 | Type 4 | S | 6.5 | 25.0 | Complete remission | |
| 4 | Male | 46 | 4 | 12 | Type 4 | DI | 5.0 | 10.0 | Complete remission | |
| 5 | Male | 58 | 3 | 360 | Type 4 | DI | 7.0 | 18.0 | Complete remission | |
| 6 | Male | 49 | Diabetes mellitus | 5 | 8 | Type 4 | DI | 5.0 | 16.0 | Complete remission |
| 7 | Male | 54 | 2 | 6 | Type 4 | DI | 6.0 | 23.0 | Complete remission | |
| 8 | Male | 62 | 5 | 15 | Type 4 | S | 6.0 | 28.0 | Complete remission | |
| 9 | Male | 50 | 2 | 6 | Type 3 | S+DI | 6.0 | 15.0 | Complete remission | |
| 10 | Male | 49 | 4 | 21 | Type 3 | S | 5.8 | 25.0 | Complete remission | |
| 11 | Male | 34 | 4 | 7 | Type 4 | S | 5.0 | 24.0 | Complete remission | |
| 12 | Male | 71 | Diabetes mellitus | 2 | 6 | Type 4 | DI | 7.0 | 25.0 | Complete remission |
| 13 | Female | 37 | 6 | 18 | Type 3 | S+DI | 7.0 | 20.0 | No improvement | |
| 14 | Male | 42 | Ulcerative colitis | 3 | 10 | Type 3 | DI | 6.0 | 29.0 | Complete remission |
| 15 | Male | 55 | 10 | 360 | Type 4 | S+DI | 5.0 | 26.0 | Complete remission | |
| 16 | Male | 40 | 10 | 21 | Type 3 | S+DI | 6.1 | 23.0 | Complete remission | |
| 17 | Male | 41 | 3 | 7 | Type 3 | DI | 6.2 | 28.0 | Complete remission | |
| 18 | Male | 38 | Diabetes mellitus | 5 | 19 | Type 4 | DI | 6.8 | 25.0 | Complete remission |
| 19 | Male | 44 | 3 | 15 | Type 3 | S+DI | 6.9 | 32.0 | Complete remission | |
| 20 | Male | 42 | 2 | 8 | Type 2 | S+DI | 7.0 | 13.0 | Complete remission | |
| 21 | Male | 41 | 8 | 68 | Type 3 | S | 5.1 | 29.0 | Partial improvement | |
| 22 | Male | 28 | 1 | 6 | Type 2 | DI | 5.2 | 20.0 | Complete remission |
REFERENCES
1. Dudukgian H, Abcarian H. Why do we have so much trouble treating anal fistula? World J Gastroenterol 2011;17:3292–6.



2. Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–90.


3. Garcia-Olmo D, Herreros D, Pascual M, Pascual I, De-La-Quintana P, Trebol J, et al. Treatment of enterocutaneous fistula in Crohn’s disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis 2009;24:27–30.



4. Borowski DW, Gill TS, Agarwal AK, Bhaskar P. Autologous adipose-tissue derived regenerative cells for the treatment of complex cryptoglandular fistula-in-ano: a report of three cases. BMJ Case Rep 2012;2012:bcr2012006988.



5. Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 2013;31:2575–81.



6. Laureti S, Gionchetti P, Cappelli A, Vittori L, Contedini F, Rizzello F, et al. Refractory complex Crohn’s perianal fistulas: a role for autologous microfragmented adipose tissue injection. Inflamm Bowel Dis 2020;26:321–30.



7. Dige A, Hougaard HT, Agnholt J, Pedersen BG, Tencerova M, Kassem M, et al. Efficacy of injection of freshly collected autologous adipose tissue into perianal fistulas in patients with Crohn’s disease. Gastroenterology 2019;156:2208–16.


8. Naldini G, Sturiale A, Fabiani B, Giani I, Menconi C. Micro-fragmented adipose tissue injection for the treatment of complex anal fistula: a pilot study accessing safety and feasibility. Tech Coloproctol 2018;22:107–13.



10. Corman ML. Colon and rectal surgery. Lippincott Williams & Wilkins; 2005.
11. Beck DE, Roberts PL, Saclarides TJ, Senagore AJ, Stamos MJ, Wexner SD. The ASCRS textbook of colon and rectal surgery. 2nd ed. Springer; 2011.

12. Vogel JD, Johnson EK, Morris AM, Paquette IM, Saclarides TJ, Feingold DL, et al. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum 2016;59:1117–33.


13. Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn’s disease. Gastroenterology 2003;125:1508–30.


14. Frenkel J, Orkin B, Fischkoff K, Young H. A new classification system for perirectal fistulas. In: ASCRS annual meeting abstracts. p. A32–3. 2002 ASCRS Annual Meeting; 2002 Jun 3–8; Chicago, IL, USA. Dis Colon Rectum 2002;45:581-2.

15. Bakhtawar N, Usman M. Factors increasing the risk of recurrence in fistula-in-ano. Cureus 2019;11:e4200.



16. Mei Z, Wang Q, Zhang Y, Liu P, Ge M, Du P, et al. Risk factors for recurrence after anal fistula surgery: a meta-analysis. Int J Surg 2019;69:153–64.


17. Jordán J, Roig JV, García-Armengol J, García-Granero E, Solana A, Lledó S. Risk factors for recurrence and incontinence after anal fistula surgery. Colorectal Dis 2010;12:254–60.


18. The Surgisis AFP anal fistula plug: report of a consensus conference. Colorectal Dis 2008;10:17–20.


19. Lalhruaizela S. Endofistula laser ablation of fistula-in-ano: a new minimally invasive technique for the treatment of fistula-in-ano. Ann Coloproctol 2022;38:301–6.



20. Lee JL, Yoon YS, Yu CS. Treatment strategy for perianal fistulas in Crohn disease patients: the surgeon’s point of view. Ann Coloproctol 2021;37:5–15.




21. Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery: factors associated with recurrence and incontinence. Dis Colon Rectum 1996;39:723–9.


22. Emile SH. Recurrent anal fistulas: when, why, and how to manage? World J Clin Cases 2020;8:1586–91.



23. Roig JV, García-Armengol J. Treatment of complex cryptoglandular anal fistulas: does it still require an experienced surgeon? Cir Esp 2013;91:78–89.


24. Sangwan YP, Rosen L, Riether RD, Stasik JJ, Sheets JA, Khubchandani IT. Is simple fistula-in-ano simple? Dis Colon Rectum 1994;37:885–9.


25. Vial M, Parés D, Pera M, Grande L. Faecal incontinence after seton treatment for anal fistulae with and without surgical division of internal anal sphincter: a systematic review. Colorectal Dis 2010;12:172–8.


26. Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 2008;32:48–57.



27. Grasys J, Kim BS, Pallua N. Content of soluble factors and characteristics of stromal vascular fraction cells in lipoaspirates from different subcutaneous adipose tissue depots. Aesthet Surg J 2016;36:831–41.


28. Pallua N, Pulsfort AK, Suschek C, Wolter TP. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast Reconstr Surg 2009;123:826–33.


29. Jeong H, Hwang SH, Kim HR, Ryu KO, Lim J, Yu HM, et al. Effectiveness of autologous fat graft in treating fecal incontinence. Ann Coloproctol 2019;35:144–51.




30. Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg 2007;119:775–87.


31. Lee CH, Moioli EK, Mao JJ. Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. Conf Proc IEEE Eng Med Biol Soc 2006;2006:775–8.


- TOOLS








