- Search
| Ann Coloproctol > Volume 39(6); 2023 > Article |
|
Abstract
Purpose
Most predictive factors for lymph node metastasis in rectal neuroendocrine tumors (NETs) have been based on local and endoscopic resection. We aimed to evaluate the risk factors for lymph node metastasis in patients who underwent radical resection for rectal NETs and stratify the risk of lymph node metastasis.
Methods
Sixty-four patients who underwent radical resection for rectal NETs between January 2001 and January 2018 were included. We investigated the risk factors of lymph node metastasis using clinicopathologic data. We also performed a risk stratification for lymph node metastases using the number of previously known risk factors. For oncologic outcomes, the 5-year overall survival and recurrence-free survival were evaluated in both groups.
Results
Among the patients who underwent radical surgery, 32 (50.0%) had lymph node metastasis and 32 (50.0%) had non–lymph node metastasis. In the multivariable analysis, only the male sex was identified as a risk factor for lymph node metastasis (odds ratio, 3.695; 95% confidence interval, 1.128–12.105; P=0.031). When there were 2 or more known risk factors, the lymph node metastasis rate was significantly higher than when there were one or no risk factors (odds ratio, 3.667; 95% confidence interval, 1.023–13.143; P=0.046). There was also no statistical difference between the 2 groups in 5-year overall survival (P=0.431) and 5-year recurrence-free survival (P=0.144).
The incidence of rectal neuroendocrine tumors (NETs) is rapidly increasing as colonoscopy is frequently used as a screening test for colorectal cancer [1, 2]. Rectal NETs grow slowly and have a benign course as compared to adenocarcinoma. Most rectal NETs are less than 10 mm in size and are easily removed by endoscopic treatment resulting in a good prognosis [3]. However, in 2010, the World Health Organization (WHO) and American Joint Cancer Commission (AJCC) classified it as a malignant tumor that can invade and metastasize to other organs [4]. The prognosis of patients with regional or distant metastases is similar to that of patients with adenocarcinoma [5]. According to the Surveillance, Epidemiology, and End Results (SEER) database, the 5-year survival rates of rectal NETs from 1992 to 1999 were 90.8% for localized, 48.9% for regional metastasis, and 32.3% for distant metastasis [6].
A crucial aspect of the treatment strategy is the risk assessment for lymph node metastasis, which determines whether a local or radical resection is needed [7]. Several studies have investigated the risk factors for lymph node metastasis in rectal NETs. According to previous studies, tumor size, stage, depth of invasion, angiolymphatic invasion (ALI), differentiation, and margin status are risk factors that are considered in determining treatment options [8]. However, most of these risk factors were determined by local resection, including endoscopy, and imaging was used to determine lymph node metastasis.
Therefore, we aimed to evaluate the risk factors and risk stratification for lymph node metastasis in patients who underwent radical resection for rectal NETs. We also evaluated the long-term outcomes of radical surgery for rectal NETs.
This study was approved by the Institutional Review Board of the National Cancer Center (No. NCC2020-0154). The need for informed consent was waived due to the retrospective study design.
We retrospectively reviewed the medical records of patients diagnosed with rectal NETs at the National Cancer Center (Goyang, Korea) between January 2001 and January 2018. Patients with concurrent sigmoid, rectosigmoid, rectal adenocarcinomas, or distant metastasis of NETs were excluded. Clinical data extracted from the medical records included age, sex, endoscopic size, distance from the anal verge, follow-up period, type of treatment, and recurrence. Histopathological evaluation was performed to determine the depth of invasion, ALI, and Ki-67 index. Tumor size was defined as the largest diameter recorded in the pathologic report. The rectum was within 15 cm of the anal verge on colonoscopy, and tumor distance was defined as the distance from the anal verge to the tumor. Lymph node metastasis was confirmed in the pathological report after radical surgery. After surgery, recurrence was defined as regional when it occurred in the lymph nodes around the lesion and distant when it occurred in other organs.
We divided the patients into 2 groups: lymph node metastasis and non–lymph node metastasis. The risk factors for lymph node metastasis in patients who underwent radical surgery for rectal NETs were studied using clinicopathologic data. We also performed risk stratification for lymph node metastases in patients with histopathologically confirmed lymph node metastasis after radical resection. Patients were divided into groups according to the number of previously known risk factors: pathologic tumor size ≥10 mm, ALI, and muscularis propria invasion.
Overall survival (OS) and recurrence-free survival (RFS) were evaluated to study the oncologic outcomes. The OS was defined as the time from the date of diagnosis to the date of death. RFS was defined as the time from diagnosis to recurrence. Survival data were censored on the date of the last follow-up.
The baseline characteristics of the patients were summarized in terms of frequency and percentage for categorical variables and median and range (minimum to maximum) for continuous variables. The 2 groups were compared using the chi-square test or Fisher exact test for categorical variables and the 2-sample t-test or Wilcoxon rank sum test for continuous variables. To confirm the risk factors for lymph node metastasis, a multivariable model was created using a factor of P<0.05 in the univariable logistic model or previously known risk factors, and then the final multivariable model was selected using the elimination criterion (P>0.05). Survival curves were estimated using the Kaplan-Meier method, and survival differences were tested using the log-rank test. Statistical significance was set at P<0.05. All statistical analyses were conducted using R ver. 4.1.2 (R Foundation for Statistical Computing).
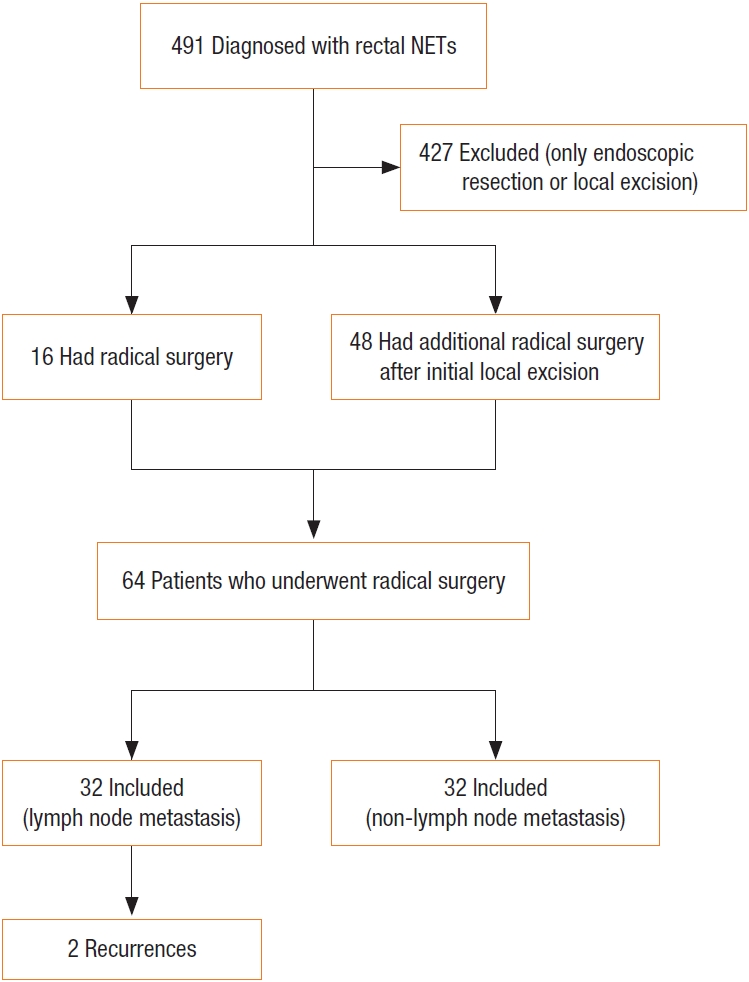
A total of 491 patients were diagnosed with rectal NETs at our institute. Among them, 16 patients underwent primary radical surgery, and 48 underwent additional surgery after initial endoscopic resection or transanal excision. The final number of patients included in the study was 64. Patients were divided into 2 groups according to the presence or absence of lymph node metastasis: 32 patients in the group with lymph node metastasis and 32 in the group without lymph node metastasis (Fig. 1).
The clinicopathologic characteristics of both groups are shown in Table 1. The median age was 53.5 years (range, 19–68 years) in the non–lymph node metastasis group and 48 years (range, 25–72 years) in the lymph node metastasis group, without statistical significance (P=0.291). There were more men in the lymph node metastasis group than in the non–lymph node metastasis group (27 [84.4%] vs. 19 [59.4%], P=0.026). There were no significant differences between the 2 groups in pathologic characteristics, including ALI (P=0.822), tumor size (P=0.193), depth of invasion (P=0.257), and Ki-67 index (P=0.107). Two recurrences were identified in the lymph node metastasis group without significant differences compared with the non–lymph node metastasis group.
In the univariate analysis, only sex was found to be significantly different between the 2 groups (P=0.026). Previously known risk factors such as tumor size, depth of invasion, and ALI were included in the multivariable analysis for the risk factors of lymph node metastasis (Table 2). Only the male sex was identified as a risk factor for lymph node metastasis (odds ratio, 3.695; 95% confidence interval, 1.128–12.105; P=0.031).
Although we could not identify the pathologic risk factors for lymph node metastasis in rectal NETs, we investigated the lymph node metastasis rate according to the number of previously known risk factors. As the number of risk factors increased, the rate of lymph node metastasis also increased (Table 3). If there were 2 or more risk factors, the lymph node metastasis rate was significantly higher than when there was less than 2 risk factor (odds ratio, 3.667; 95% confidence interval, 1.023–13.143; P=0.046) (Fig. 2).
The median follow-up period was 70 months (range, 1.64–160.31 months). The 5-year OS was 100% in both lymph node metastasis and non-lymph node metastasis groups, with no statistical difference (P=0.431). The 5-year RFS rates were 100% in the non-lymph node metastasis group and 93.2% in the lymph node metastasis group. There was also no statistically significant difference between the 2 groups (P=0.144) (Fig. 3).
In this study, we investigated the risk factors for lymph node metastasis in patients who underwent radical resection of rectal NETs. Our results showed that the male sex is a clinical risk factor for lymph node metastasis. However, we were not able to determine the pathologic risk factors associated with lymph node metastasis. Instead, we found that as the number of these risk factors increased, the rate of lymph node metastasis also increased. It was found that lymph node metastasis increased significantly when there were 2 or more risk factors.
In previous studies, due to the low number of patients who underwent radical surgery for rectal NETs, most cases of lymph node metastasis were determined by imaging studies at the time of diagnosis [2, 9]. Lymph node metastasis was diagnosed when the lymph node was round larger than 8 mm, ovoid in shape, larger than 10 mm, and when morphology showed speculations, irregular margins, and heterogeneous features. However, in our study, among 32 patients with lymph node metastasis, only 5 (15.6%) showed possible lymph node metastasis on preoperative imaging, implying that lymph node metastasis should eventually be confirmed pathologically. Therefore, our study included patients with histologically confirmed lymph node metastasis, and although it was a relatively small sample size, the incidence of lymph node metastasis was 32 out of 64 (50.0%). In previous studies, the rate of lymph node metastasis in patients who underwent radical surgery was reported to be 47.2% to 54.8% [2, 9], which is similar to our results. Most patients undergoing radical surgery for rectal NETs have at least 1 previously known risk factor for lymph node metastasis. We analyzed the correlation between the number of risk factors and lymph node metastasis and found that the rate of lymph node metastasis increased significantly when 2 or more risk factors were present. It is necessary to confirm this finding through a study with a larger sample size.
It has been reported that the rate of lymph node metastasis is 10% when the rectal NETs are 1 to 2 cm in size and 80% to 100% if >2 cm [5, 10]. Accordingly, in previous studies, tumor size greater than 1 cm has been considered a risk factor for lymph node metastasis. However, there is still no consensus on whether local resection, including endoscopic resection or radical surgery, should be performed for rectal NETs of this size. In our study, 11 patients had tumors >1 cm in size without other risk factors, and the lymph node metastasis rate was 37.5%. This suggests that surgery is recommended for tumors >1 cm in size. Ricci et al. [11] performed receiver operating characteristic curve analysis in their study and found that the cutoff value for predicting nodal involvement was 11.5 mm. However, even small rectal NETs <1 cm in size are not without the possibility of lymph node metastasis. Soga [12] reviewed 777 rectal NETs and found that the metastasis rate was 9.7% in rectal NETs < 10 mm. In our study, 4 of 6 patients with rectal NETs <10 mm without other risk factors had lymph node metastasis. This implies that the rate of lymph node metastasis may be higher, even in small-sized rectal NETs. In our study, the cutoff value for predicting lymph node metastasis was 9 mm (Supplementary Fig. 1). We suggest that a more precise criteria should be made for tumor size requiring radical surgery.
In our study, the male sex was found to be an independent risk factor for lymph node metastasis. Rectal NETs are known to occur predominantly in men [13], and this is thought to be due to racial differences [14]. However, to date, there have been no reports on the relationship between sex and lymph node metastasis. Similar to our study, Concors et al. [2] showed that the male sex was an independent risk factor for distant metastasis. We decided not to include male patients in the lymph node risk stratification because we did not find any explanation for our results. However, in addition to known pathological risk factors, we suggest that it could have a clinical impact on lymph node metastasis.
The strength of this study was the identification of risk factors in patients who underwent radical surgery for rectal NETs, which was histologically confirmed as lymph node metastasis. In addition, we evaluated whether the number of factors affected the rate of lymph node metastasis by combining previously known risk factors, which have not been attempted in previous studies. The rate of lymph node metastasis increased significantly when 2 or more risk factors were present. A large-scale cohort study is required to confirm our results.
This study has several limitations. First, the sample size was small because it only included subjects who underwent surgery. In addition, most of these subjects were high-risk patients with 1 or more risk factors for lymph node metastasis. Therefore, it is possible that confounding factors greatly influenced the determination of independent risk factors for lymph node metastasis. Therefore, we stratified the rate of lymph node metastasis by combining the previously known risk factors and determined the risk of lymph node metastasis according to the number of known risk factors. Second, as a retrospective study, an accurate analysis was impossible because some data, such as mitotic count or tumor grade, were unavailable. Third, as a single-center study, it is still difficult to apply it to clinical practice. Although a relatively large number of cases with rectal NETs were included in this study, further multicenter studies are needed to have more statistical power. We are currently collecting the data of patients who underwent radical surgery for rectal NETs from multiple institutes. Our present study could provide the direction for future multicenter studies analyzing lymph node metastasis for rectal NETs. Lastly, despite the long-term follow-up periods, there were few events of death or recurrence, making it difficult to analyze the prognostic factors. However, we found that, even if lymph node metastasis was present, favorable oncologic outcomes were observed when surgery was performed.
In conclusion, we found that the rate of lymph node metastasis increased significantly when the number of known risk factors is 2 or more. Favorable oncological outcomes have been observed in patients who undergo surgery, even those with lymph node metastasis.
Notes
Funding
This study was supported by a grant from the National Cancer Center of Korea (No. NCC-2110230).
Author contributions
Conceptualization: MJJ, SSP; Data curation: MJJ, SSP; Formal analysis: MJJ, SSP, DEL, KY; Funding acquisition: BCK; Investigation: MJJ, SSP; Methodology: MJJ, SSP; Project administration: MJJ, SSP; Resources: SSP, SCP, HJC, CWH, DKS, KSH, BK, BCK, JHO; Software: MJJ, SSP, DEL, KY; Supervision: SSP, BCK; Validation: BCK; Visualization: MJJ, SSP; Writing–original draft: MJJ, SSP; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Supplementary materials
Supplementary Fig. 1. Cutoff value of tumor size for lymph node metastasis.
Supplementary materials are available from https://doi.org/10.3393/ac.2022.00913.0130.
Fig. 2.
Forest plot of rate of lymph node metastasis according to the number of previously known risk factors. OR, odds ratio; CI, confidence interval.
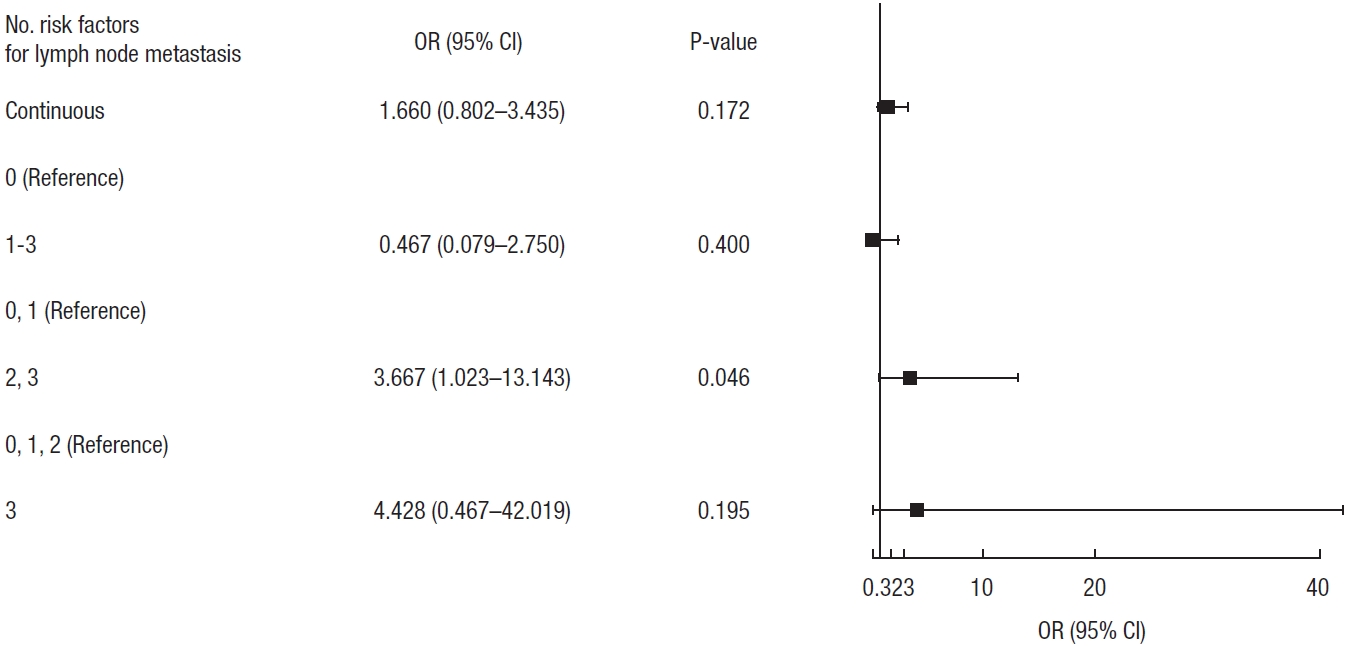
Fig. 3.
(A) Overall survival and (B) recurrence-free survival in the lymph node metastasis and the non-lymph node metastasis group.

Table 1.
Comparison of clinicopathologic characteristics between the lymph node metastasis group and non–lymph node metastasis group who underwent radical resection procedure (n=64)
Table 2.
Logistic regression analysis for the risk factor for lymph node metastasis in rectal neuroendocrine tumor (n=64, event=32)
Table 3.
Lymph node metastasis according to previously known risk factors
REFERENCES
1. Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009;41:162–5.


2. Concors SJ, Sinnamon AJ, Folkert IW, Mahmoud NN, Fraker DL, Paulson EC, et al. Predictors of metastases in rectal neuroendocrine tumors: results of a national cohort study. Dis Colon Rectum 2018;61:1372–9.


3. Kim J, Kim JH, Lee JY, Chun J, Im JP, Kim JS. Clinical outcomes of endoscopic mucosal resection for rectal neuroendocrine tumor. BMC Gastroenterol 2018;18:77.




4. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. editors. AJCC cancer staging manual. 7th ed. Springer; 2010.

5. Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H, et al. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 2007;56:863–8.



6. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59.


7. Cha JH, Jung DH, Kim JH, Youn YH, Park H, Park JJ, et al. Long-term outcomes according to additional treatments after endoscopic resection for rectal small neuroendocrine tumors. Sci Rep 2019;9:4911.




8. Sohn B, Kwon Y, Ryoo SB, Song I, Kwon YH, Lee DW, et al. Predictive factors for lymph node metastasis and prognostic factors for survival in rectal neuroendocrine tumors. J Gastrointest Surg 2017;21:2066–74.



9. Colonoscopy Study Group of Korean Society of Coloproctology. Clinical characteristics of colorectal carcinoid tumors. J Korean Soc Coloproctol 2011;27:17–20.



10. Sohn DK. Current issues involving the treatment of small rectal carcinoid tumors. J Korean Soc Coloproctol 2012;28:176–7.



11. Ricci AD, Pusceddu S, Panzuto F, Gelsomino F, Massironi S, De Angelis CG, et al. Assessment of the risk of nodal involvement in rectal neuroendocrine neoplasms: the NOVARA score, a multicentre retrospective study. J Clin Med 2022;11:713.



12. Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 2005;103:1587–95.