- Search
| Ann Coloproctol > Volume 40(1); 2024 > Article |
|
Abstract
Anal fistulas, especially complex and high fistulas, are difficult to manage. The transanal opening of the intersphincteric space (TROPIS) procedure was first described in 2017, and a high success rate of over 90% was reported in high complex fistulas. Since then, more studies and even a meta-analysis have corroborated the high efficacy of this procedure in high fistulas. Conventionally, the main focus was to close the internal (primary) opening for the fistula to heal. However, most complex fistulas have a component of the fistula tract in the intersphincteric plane. This component is like an abscess (sepsis) in a closed space (2 muscle layers). It is a well-known fact that in the presence of sepsis, healing by secondary intention leads to better results than attempting to heal by primary intention. Therefore, TROPIS is the first procedure in which, instead of closing the internal opening, the opening is widened by laying open the fistula tract in the intersphincteric plane so that healing can occur by secondary intention. Although the drainage of high intersphincteric abscesses through the transanal route was described 5 decades ago, the routine utilization of TROPIS for the definitive management of high complex fistulas was first described in 2017. The external anal sphincter (EAS) is completely spared in TROPIS, as the fistula tract on either side of the EAS is managed separately—inner (medial) to the EAS by laying open the intersphincteric space and outer (lateral) to the EAS by curettage or excision.
Managing high complex fistulas is challenging, and no gold standard or satisfactory treatment is available. Conventionally, the procedures associated with a higher success rate had higher risks of incontinence, and sphincter-sparing procedures had a low success rate in high complex fistulas [1]. Several new sphincter-saving procedures, such as the over-the-scope clip (OTSC), anal fistula plug, fistula laser closure (FiLaC), fixcision, and video-assisted anal fistula treatment (VAAFT), have been proposed in the last 2 decades [1]. These procedures are as expensive as they are device dependent. The main intention behind innovating these procedures was to find a sphincter-saving procedure for high fistulas, as fistulotomy was gold standard for low fistulas and offered 95% to 100% healing rates [1]. However, these new sphincter-saving procedures had cure rates of 40% to 70% in low simple fistulas, and no study to date has analyzed their efficacy in a cohort of exclusively high complex fistulas. Against this background, a new procedure, transanal opening of the intersphincteric space (TROPIS), first described in 2017 [2], completely preserves the external anal sphincter (EAS) and has shown highly encouraging results in high complex fistulas (85% to 94% in various studies) [3–7] (Fig. 1).
This study was reviewed and approved by the Indus International Hospital-Institute Ethics Committee (IIH-IEC) (No. EC/IIH-IEH/SP6). All study participants, or their legal guardians, provided informed written consent prior to study enrollment.
The TROPIS procedure is based on 2 principles.
It is known that in the presence of sepsis, healing by secondary intention leads to better results than healing by primary intention. The fistula tract in the intersphincteric plane is like an abscess (sepsis) in a closed space (2 sphincter muscle layers). Therefore, in TROPIS, instead of closing the internal opening primarily, it is freshened by laying open the fistula tract in the intersphincteric plane through the transanal route so that healing can occur by secondary intention (Figs. 2–7).
The EAS is completely spared in TROPIS as the fistula tract on either side of EAS is managed separately—inner (medial) to the EAS by laying open the intersphincteric space and outer (lateral) to the EAS by curettage or excision. TROPIS has no significant negative impact on continence, as the EAS is completely spared. It has been shown that the effects of partial division of the internal anal sphincter (IAS), as happens in TROPIS, can be fully compensated by performing Kegel (pelvic floor) exercises in the postoperative period.
Fistulas are divided into high and low fistulas [8]. TROPIS is indicated in high fistulas (involvement of more than 1/3 of the EAS). The high fistulas are high transsphincteric fistulas, suprasphincteric, extrasphincteric, or supralevator fistulas, high transsphincteric fistulas with an extensive (e.g., intersphincteric horseshoe fistulas) or minimal intersphincteric component, high transsphincteric fistulas with an associated abscess and high acute anorectal abscess (high transsphincteric or intersphincteric). In low fistulas (involvement of less than 1/3 of the EAS), TROPIS can be done as an EAS-sparing procedure, although fistulotomy is the gold standard in these fistulas.
There is no absolute contraindication, but the TROPIS procedure may be avoided in simple, low fistulas, since fistulotomy yields a much higher cure rate in these fistulas.
Preoperative magnetic resonance imaging (MRI) helps to accurately delineate the internal opening and the fistula tract in the intersphincteric space (Figs. 2, 4, 6). In TROPIS, the fistula tracts on both sides of the EAS are separately dealt with, and the EAS is not cut at all (Fig. 1). A curved artery forceps is inserted in the fistula tract in the intersphincteric space through the internal opening (Fig. 7A). The mucosa and the internal sphincter overlying the artery forceps are then incised with electrocautery. The edges of the resulting wound are trimmed to create a saucer-shaped wound to ensure healing by secondary intention (Figs. 3, 5, 7). A gutter is made inferiorly from the opened-up intersphincteric space to the anal verge to facilitate drainage from the intersphincteric space wound in the postoperative period (Figs. 3, 5, 7). There are no upper limits of the intra-anal wound that can be described, as the main aim is to lay open and deroof the fistula tract in the intersphincteric plane. Therefore, the resulting wound depends on the direction of the fistula tract in the intersphincteric plane. Usually, the intra-anal wound is not very high, as the internal opening is almost always at the level of the dentate line. The final wound is usually more horizontal than vertical (inferno-superior) (Figs. 3, 5, 7).
When there are multiple internal openings, the primary opening (usually in the posterior or anterior midline at the dentate line) is dealt with by TROPIS, whereas the additional internal opening (even the supralevator) can be simply cauterized. It has been shown that microorganisms usually have ingress into the fistula through the primary opening at the dentate line (high-pressure zone), while the additional internal openings are either formed by a high-pressure intersphincteric abscess bursting into the rectum or are formed iatrogenically. Once the primary opening at the dentate line heals, the additional internal openings usually heal subsequently.
As mentioned, the range of “laying open” depends on the direction and extent of the fistula tract in the intersphincteric space (intersphincteric tract). Therefore, the depth of the wound is usually from the anal mucosa to the intersphincteric space (the mucosa and the IAS are incised). The center of the wound is at the primary opening. The aim should be to lay open the entire intersphincteric tract, but in cases with long fistula tracts in the intersphincteric space, as in intersphincteric horseshoe fistulas (Fig. 6), it is not necessary to lay open the entire total intersphincteric tract. Laying open (deroofing) a sufficient tract so that the complete intersphincteric tract remains empty by persistent drainage through the laid-open wound in the postoperative period also suffices and leads to good healing.
The fistula tract lateral to (outside) the EAS (fistula tract in the ischioanal fossa) is dealt with such that no fluid or pus accumulates there in the postoperative period (curettage and placement of a drainage tube, excision, or laser ablation). We prefer to curette the tracts and place a drainage tube sutured to the perianal skin (Figs. 3, 5, 7). The drainage is placed lateral to the EAS but does not cross the EAS. The drainage tube is removed once the intra-anal TROPIS wound has completely healed according to a clinical assessment. As the intra-anal wound heals in most patients by 3 months after surgery, we set a period of 3 months postoperatively to remove the drainage tube. As a personal preference, we also perform postoperative MRI at 3 months to confirm radiological healing before taking out the tube, as almost all of these fistulas are complex high fistulas (Figs. 2, 4, 6). However, the primary tube removal criterion is complete intra-anal wound healing clinically (preferably radiologically), not the passage of 3 months.
In the postoperative period, everyday activities and resumption of daily routine are started from the day after surgery. A gentle per rectal examination is done once a day for the first 4 weeks to separate the wound edges of the intra-anal TROPIS wound and prevent them from sticking together. This is important because the aim is to achieve healing of the laid-opened (deroofed) fistula tract in the intersphincteric plane by secondary intention.
The complications in the postoperative period are occasional bleeding from the intra-anal wound, especially after the per rectal examination. The bleeding stopped spontaneously in most patients, and only 2 out of 650 patients had to be taken to the operating room to suture-ligate the bleeder present in the wound edge. The tube sutured to the perianal skin (Figs. 3, 5, 7) came out before 3 months in a few patients because it cut the sutures through the skin. The draining tube was reinserted and stitched again to the perianal skin in the outpatient department.
The TROPIS procedure is a paradigm shift in understanding and managing complex anal fistulas [9, 10]. It is perhaps the first procedure that challenges the current practice of attempting to close the internal opening to heal complex high fistulas, as is done in advancement flaps, VAAFT, anal fistula plugs, OTSC, FiLaC, and so forth. The idea and concept of transanally laying open the fistula tract in the intersphincteric space arouse suspicion and apprehension that widening the internal opening might facilitate the passage of fecal matter into the fistula tract, thereby worsening the disease. However, this does not happen because the fistula tract in the EAS is not widened in the TROPIS procedure. The EAS muscle's tone prevents fecal matter from passing into the fistula tract in most cases. Moreover, the high success rate of TROPIS (85%–93%) in high complex fistulas, even in long-term follow-up, allays this fear.
We published our results in an exclusive cohort of high complex fistulas in 2017 (90.4% success rate in 52 patients) [11], in 2018 (84.6% success rate in an exclusive cohort of 26 supralevator anal fistulas) [2, 12], in 2021 (87.6% in 325 patients, 86.0% in 408 patients) [13], and in 2023 (86.4% in 650 patients) [3]. In 2022, Li et al. [4] reported a success rate of 86.5% in 41 patients with high fistulas, and in 2021, Huang et al. [5] published a success rate of 93.7% in 48 fistula patients. A recent meta-analysis [6] highlighted that of all sphincter-sparing procedures utilized to treat high fistulas, TROPIS has the highest success rate.
The TROPIS procedure has a minimal impact on continence [4–6, 13]. Although the mean incontinence scores deteriorated after TROPIS, these changes were insignificant [4, 13]. The increase in the number of patients with incontinence after TROPIS ranged from 0% [5] to 2% [4, 13], and the reported incontinence was mainly gas and urge incontinence [13]. Even this minor deterioration in continence was corrected and fully compensated by doing Kegel (pelvic floor) exercises in the postoperative period [13]. The reason for this could be that the IAS is responsible for maintaining resting anal pressure (tone), while EAS is mainly responsible for squeezing or peak anal pressure. The slight deterioration in resting anal pressure due to the partial division of the IAS is perhaps compensated by strengthening of EAS by Kegel exercises [13].
The routine drainage of high intersphincteric and supralevator abscesses through the transanal route was described 5 decades ago. Still, it had never been advocated for the definitive management of abscesses or fistulas. The routine utilization of TROPIS in the definitive management of high complex fistulas was first described by us in 2017 [11]. The TROPIS procedure has been demonstrated to have a high success rate in high transsphincteric, suprasphincteric (Figs. 2, 3), supralevator, and horseshoe fistulas (Figs. 6, 7). It has also been shown to be effective for the definitive single-stage management of fistulas associated with acute anorectal abscesses [13].
The sphincter complex (IAS and EAS) has different dimensions in different positions of the anal canal. The sphincter complex and intersphincteric plane are quite well-developed posteriorly, slightly less developed laterally, and least developed anteriorly. The sphincter complex in the anterior anal region in female patients is even more attenuated. Therefore, while performing the TROPIS procedure in anterior fistulas, caution must be exercised to avoid damaging the EAS. It needs to be kept in mind that due to the poorly developed intersphincteric space, anterior fistulas in females are more often totally transsphincteric and have minimal or no intersphincteric component. Therefore, the TROPIS procedure may not be possible in many anterior fistulas, especially in female patients.
Few procedures similar to the TROPIS procedure have been described in the past. However, there are significant differences between them and the TROPIS procedure. Parks [7] described a procedure that involved the limited division of the lower half of the IAS to drain the infected anal gland, in addition to coring out or curetting the outer portion of the fistula tract. This procedure was replicated by Sumikoshi et al. [8] and was further developed by Tsuji et al. [14]. A possible shortcoming of Parks procedure was limiting the internal sphincterotomy to below the dentate line. Cases in which an infected anal gland or part of its duct is located at a higher plane in the intersphincteric space might be missed, leading to a high risk of recurrence. In the TROPIS procedure, the internal anal sphincterotomy is extended as high as the tract goes up in the intersphincteric space to ensure eradication of the origin of infection. There is also a conceptual difference between the 2 procedures. The primary aim of Parks procedure was to remove the infected anal gland, whereas in the TROPIS procedure, the aim is both to remove the infected anal gland and to lay open the fistula tract in the intersphincteric space, allowing it to heal by secondary intention. Deroofing the fistula tract in the intersphincteric space is perhaps the easiest way to tackle the sepsis there. In Parks procedure, the infected anal gland is removed, but the sepsis in the intersphincteric space is not completely eradicated, as in intersphincteric horseshoe fistulas. This residual sepsis would increase the chances of recurrence. Therefore, the internal sphincterotomy is higher and wide in the TROPIS procedure than in Parks procedure. In Hanley procedure, the EAS is divided, unlike the TROPIS procedure. Goligher [15] developed a procedure that combined the advantages of Parks procedure and Hanley procedure, but as in Parks procedure, the sepsis in the intersphincteric space (other than the primary infected gland) was not taken care of. Ligation of the intersphincteric tract (LIFT) deals with the infected anal gland and the sepsis in the intersphincteric space. However, unlike the TROPIS procedure in which healing in the infected intersphincteric fistula tract is allowed to occur by secondary intention, the intersphincteric space is allowed to close by primary intention in the LIFT procedure. It is well-known that in the presence of infection, healing by secondary intention yields better results than healing by primary intention.
TROPIS has a higher success rate than LIFT (85%–93% vs. 40%–92%), although LIFT has been more widely utilized and published than TROPIS. Only time and more experience from across the globe will confirm which procedure is better, although both have advantages and disadvantages. LIFT preserves the IAS and thus does not pose any risk of incontinence. However, LIFT is technically difficult, has a longer learning curve, and is difficult to perform in high intersphincteric, suprasphincteric, and intersphincteric horseshoe fistulas. Nonetheless, both these sphincter-preserving procedures are complementary and are likely to be essential parts of the surgeon’s armamentarium to manage high complex anal fistulas.
Notes
Author contributions
Conceptualization: all authors; Data curation: all authors; Formal analysis: all authors; Investigation: all authors; Methodology: all authors; Project administration: all authors; Resources: all authors; Software: PG; Supervision: all authors; Validation: all authors; Visualization: all authors; Writing–original draft: PG; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Fig. 1.
The steps of the transanal opening of the intersphincteric space (TROPIS) procedure. EAS, external anal sphincter; IO, internal opening.
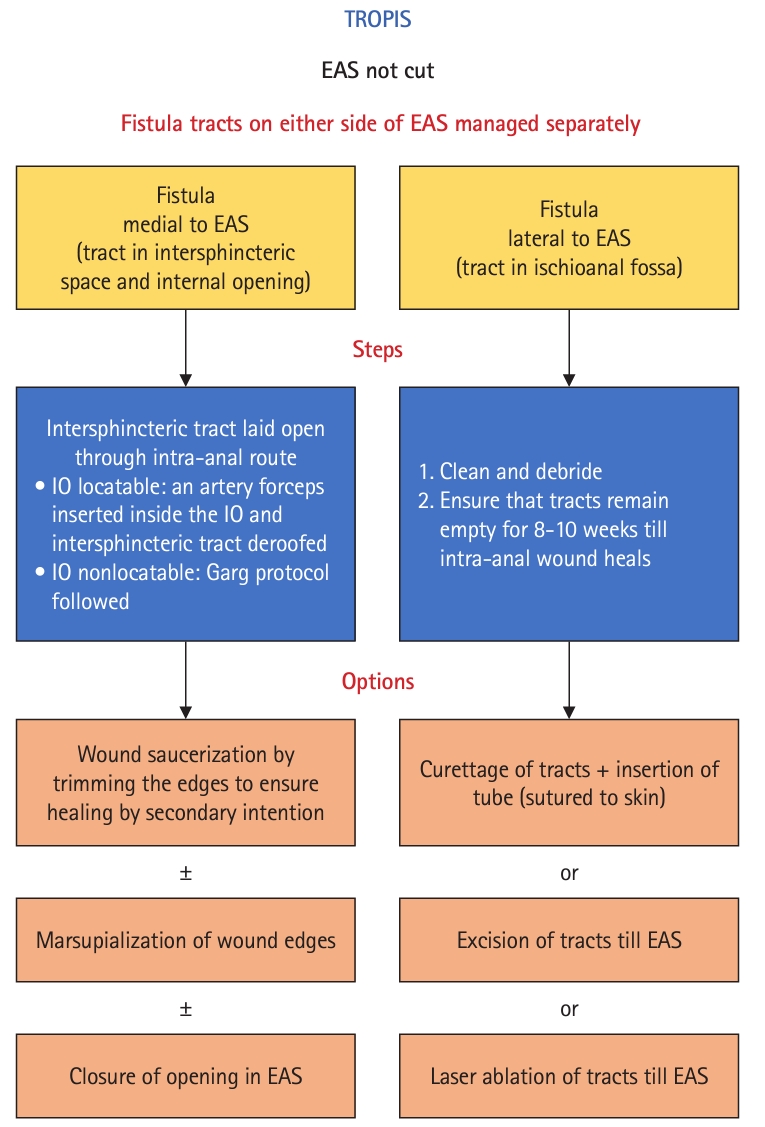
Fig. 2.
A 23-year-old male patient with left suprasphincteric abscess with no external opening. He was managed definitively in the first surgery by the transanal opening of the intersphincteric space (TROPIS) procedure. The patient had clinically healed at 3 months postoperatively, and the magnetic resonance imaging (MRI) showed that the fistula had completely healed. (A) Schematic diagram of the axial section. (B) Schematic diagram of the coronal section. (C) Preoperative MRI of the axial T2 section, showing a high internal opening at the posterior midline (arrow, fistula tract). (D) Preoperative MRI of the coronal T2 section, showing a left suprasphincteric abscess with no external opening (arrow, fistula tract). (E) Postoperative 3 months MRI of the axial T2 section, showing a completely healed fistula and the internal opening. (F) Postoperative 3 months MRI of the coronal T2 section, showing a completely healed fistula and the internal opening.

Fig. 3.
Photographs of the patient in Fig. 2, a 23-year-old male patient with a left suprasphincteric abscess with no external opening. (A) Preoperative photograph with no external opening. The blue mark on the left buttock shows the area of maximum induration. (B, C) Immediate postoperative photographs. (B) The final transanal opening of the intersphincteric space (TROPIS) wound inside the anal canal. (C) Final picture after surgery. A drainage tube in the left suprasphincteric abscess can be seen sutured to the skin. This was taken at 3 months postoperatively, after the TROPIS wound (internal opening) had healed completely.
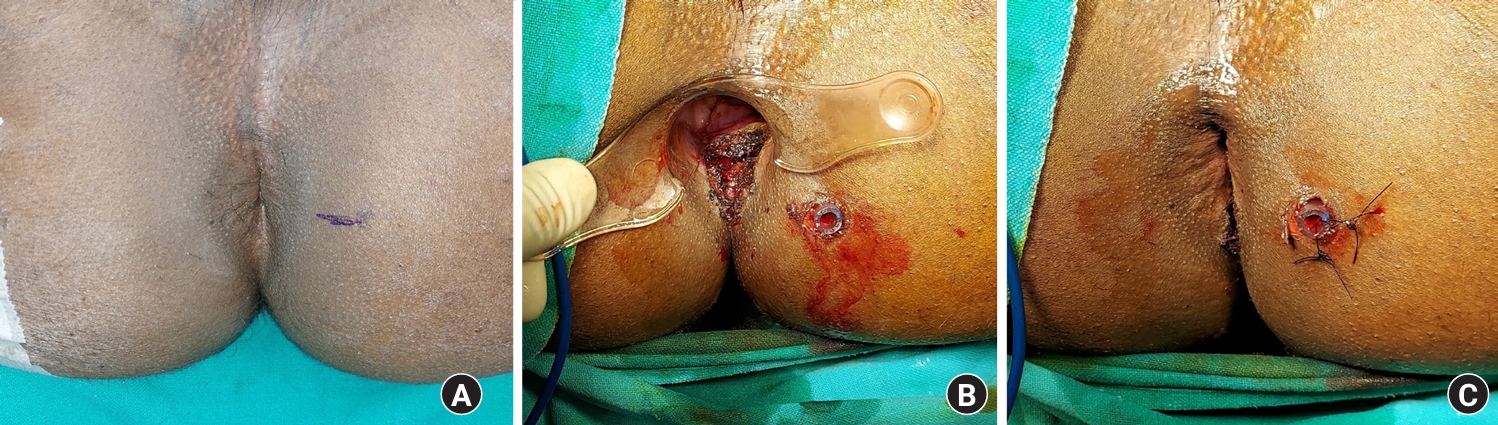
Fig. 4.
A 30-year-old male patient with recurrent right high transsphincteric fistula with multiple branches. He was managed successfully by the transanal opening of the intersphincteric space (TROPIS) procedure, and postoperative magnetic resonance imaging (MRI) at 5 months showed that the fistula had completely healed. (A) Schematic diagram of the axial section. (B) Schematic diagram of the coronal section showing a high transsphincteric fistula with an additional (second) branch extending superiorly along the inferior surface of the right levator muscle. (C) Preoperative MRI of the axial T2 section, showing a high internal opening at the posterior midline (arrow, fistula tract). (D) Preoperative MRI of the coronal short inversion time inversion recovery sequence section showing a right high transsphincteric fistula (yellow arrow) with an additional (second) branch extending superiorly along the inferior surface of the right levator muscle. The complete fistula tract from the external opening to the internal opening can be visualized (white arrows). (E) Postoperative 5 months MRI of the axial T2 section, showing a completely healed fistula and the internal opening. (F) Postoperative 5 months MRI of the coronal T2 section, showing a completely healed fistula and the internal opening.
Fig. 5.
Photographs of the patient in Fig. 4, a 30-year-old male patient with recurrent right high transsphincteric fistula. (A) Preoperative photograph showing the external opening on the right buttock. (B, C) Immediate postoperative photographs. (B) The final transanal opening of the intersphincteric space (TROPIS) wound inside the anal canal. (C) Final picture after surgery. A drainage tube in the right high transsphincteric tract can be seen sutured to the skin. This photograph was taken at 3 months postoperatively, after the TROPIS wound (internal opening) had healed completely.

Fig. 6.
A 60-year-old male patient with a high intersphincteric posterior horseshoe abscess. He was managed definitively in the first surgery by the transanal opening of the intersphincteric space (TROPIS) procedure. The patient had clinically healed at 3 months postoperatively, and magnetic resonance imaging (MRI) showed that the fistula had completely healed. (A) Schematic diagram of the axial section. (B) Schematic diagram of the coronal section. (C) Preoperative MRI of the axial short inversion time inversion recovery sequence (STIR) section, showing an intersphincteric posterior horseshoe abscess with a high internal opening at the midline posteriorly (arrows, fistula tract). (D) Preoperative MRI of the coronal T2 section, showing a high intersphincteric posterior horseshoe abscess (arrows, fistula tract). (E) Postoperative 3 months MRI of the axial STIR section, showing a completely healed abscess (and fistula) and the internal opening. (F) Postoperative 3 months MRI of the coronal T2 section, showing a completely healed fistula and the internal opening.
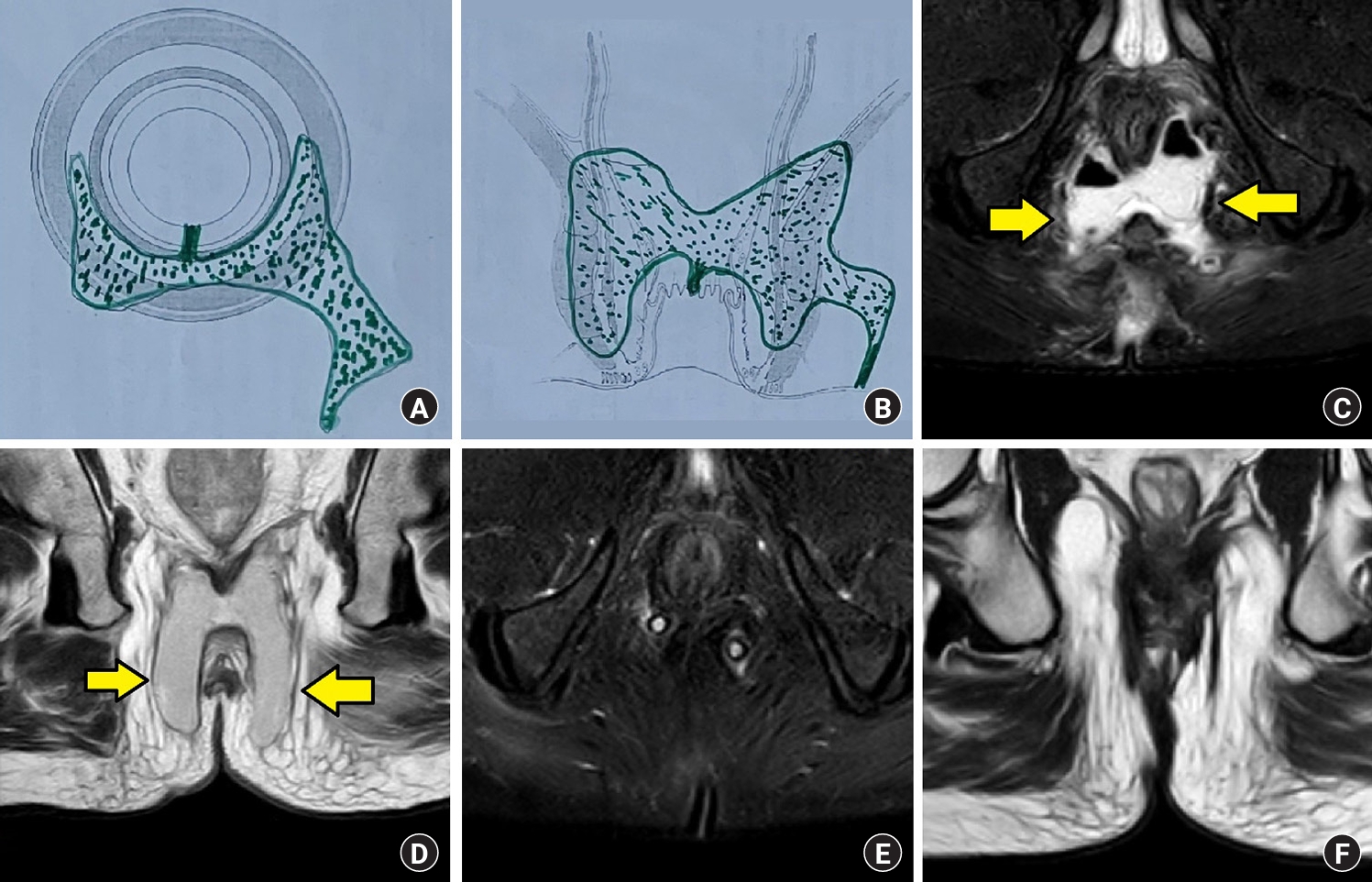
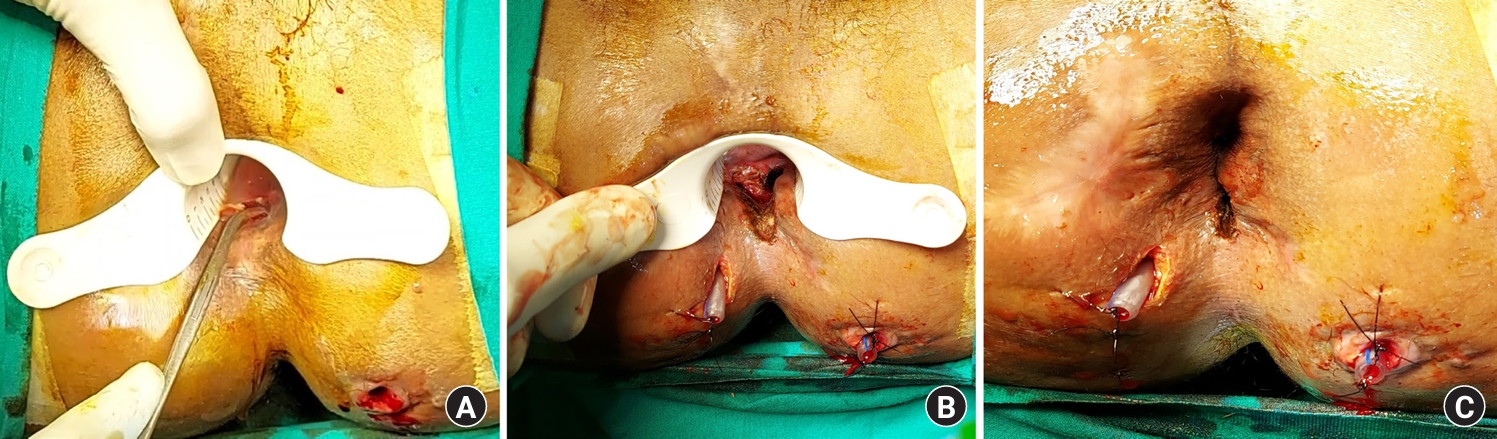
Fig. 7.
Photographs of the patient in Fig. 6, a 60-year-old male patient with a high intersphincteric posterior horseshoe abscess. (A) Intraoperative photograph showing insertion of a curved artery forceps into the fistula tract in the intersphincteric plane through the internal opening. The mucosa and the internal sphincter were incised over the forceps. (B, C) Immediate postoperative photographs. (B) The final transanal opening of the intersphincteric space (TROPIS) wound inside the anal canal. (C) Final picture after surgery. A drainage tube can be seen on each side of the horseshoe abscess. This was taken after the TROPIS wound (internal opening) had healed completely after 3 months.
REFERENCES
1. Gaertner WB, Burgess PL, Davids JS, Lightner AL, Shogan BD, Sun MY, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum 2022;65:964–85.


2. Garg P. Understanding understanding and treating supralevator fistula-in-ano: MRI analysis of 51 cases and a review of literature. Dis Colon Rectum 2018;61:612–21.


3. Garg P, Kaur B. Transanal opening of intersphincteric space: a novel procedure to manage highly complex anal fistulas. Dis Colon Rectum 2023;66:e292–3.


4. Li YB, Chen JH, Wang MD, Fu J, Zhou BC, Li DG, et al. Transanal opening of intersphincteric space for fistula-in-ano. Am Surg 2022;88:1131–6.



5. Huang B, Wang X, Zhou D, Chen S, Li B, Wang Y, et al. Treating highly complex anal fistula with a new method of combined intraoperative endoanal ultrasonography (IOEAUS) and transanal opening of intersphincteric space (TROPIS). Videosurg Other Miniinvasive Tech 2021;16:697–703.



6. Huang H, Ji L, Gu Y, Li Y, Xu S. Efficacy and safety of sphincter-preserving surgery in the treatment of complex anal fistula: a network meta-analysis. Front Surg 2022;9:825166.



8. Sumikoshi Y, Takano M, Okada M, Hiratsuka J, Sato S. Classification of fistulas. Nippon Daicho Komonbyo Gakkai Zasshi 1972;25:177–84.

9. Włodarczyk M, Włodarczyk J, Sobolewska-Włodarczyk A, Trzciński R, Dziki Ł, Fichna J. Current concepts in the pathogenesis of cryptoglandular perianal fistula. J Int Med Res 2021;49:300060520986669.



10. Ji L, Zhang Y, Xu L, Wei J, Weng L, Jiang J. Advances in the treatment of anal fistula: a mini-review of recent five-year clinical studies. Front Surg 2021;7:586891.



11. Garg P. Transanal opening of intersphincteric space (TROPIS): a new procedure to treat high complex anal fistula. Int J Surg 2017;40:130–4.


12. Garg P. Intersphincteric component in a complex fistula-in-ano is like an abscess and should be treated like one. Dis Colon Rectum 2018;61:e26.

13. Garg P, Kaur B, Menon GR. Transanal opening of the intersphincteric space: a novel sphincter-sparing procedure to treat 325 high complex anal fistulas with long-term follow-up. Colorectal Dis 2021;23:1213–24.



14. Tsuji Y, Takano S, Yamada K, Takano M. A retrospective critique of the various sphincter-preserving surgical procedures for ischiorectal fistula. J Anus Rectum Colon 2022;6:100–12.



15. Goligher JC. Surgery of the anus, rectum and colon. 3rd ed. Cassell; 1975.
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,499 View
- 184 Download
- Related articles in ACP
-
Newer procedures need to demonstrate efficacy in high complex anal fistulas2023 August;39(4)