- Search
Abstract
Purpose
The serum level of carcinoembryonic antigen (CEA) is a clinical prognostic factor in the follow-up evaluation of patients with colon cancer. We aimed to evaluate the prognostic significance of the rate of decrease of the perioperative serum CEA level in patients with colon cancer after a curative resection.
Methods
A total of 605 patients who underwent a curative resection for colon cancer between January 2000 and December 2007 were enrolled retrospectively. The rate of decrease was calculated using the following equation: ([preoperative CEA - postoperative CEA]/[preoperative CEA] ├Ś100).
Results
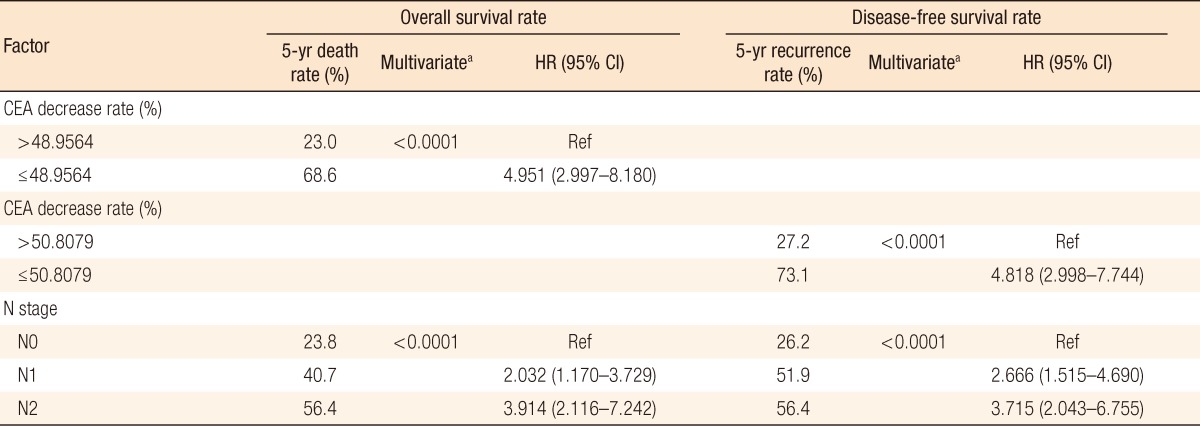
In the group with a preoperative serum CEA level of >5 ng/mL, the normalized group with a postoperative serum CEA level of Ōēż5 ng/mL showed a better overall survival (OS) rate and disease-free survival (DFS) rate than those of the non-normalized group (P Ōēż 0.0001). The "cutoff values" of the rate of decrease in the perioperative serum CEA that determined the OS and the DFS were 48.9% and 50.8%, respectively. In the multivariate analysis of preoperative serum CEA levels >5 ng/mL, the prognostic factors for the OS and the DFS were the cutoff value (P < 0.0001) and the pN stage (P < 0.0001).
Colorectal cancer (CRC) remains the leading cause of cancer-related deaths worldwide, and the TNM classification is the best prognostic predictor of outcomes in patients with CRC [1-3]. However, factors such as the TNM classification cannot be readily obtained preoperatively. In spite of the many investigations of prognostic factors in CRC, few parameters are predictive of the risk of tumor relapse in individual patients. An accurate prognostic parameter before surgery is important, and when preoperative prognostic factors or high risks of tumor relapse are present in patients with CRC, different treatment approaches are required.
Tumor markers have been applied in clinical practice for several decades, including in the diagnosis, screening, staging, and monitoring of the effects of treatments [1, 2, 4-6]. Since its initial description in 1965 by Gold and Freedman [7], carcinoembryonic antigen (CEA) has been the tumor marker investigated most extensively for CRC. Indeed, many studies have demonstrated the prognostic value of the preoperative CEA level after surgical colon resection [8-11]. However, the early postoperative decrease in the CEA level, as compared with the preoperative CEA level, as an independent predictor of disease-free survival (DFS) and overall survival (OS) in patients with colon cancer has been evaluated only rarely. Therefore, we analyzed the prognostic values by evaluating the preoperative serum CEA level and the perioperative rate of decrease in serum CEA level in patients who underwent a curative resection for colon cancer.
Between January 2000 and December 2007, 895 patients with primary colon cancer underwent a surgical resection in the Department of Surgery, Inje University Busan Paik Hospital, Inje University College of Medicine. All patients who had undergone a surgical resection for colon cancer were registered in a prospectively-collected colon-cancer database and were followed up. Six hundred five patients with pathological stages I to III tumors were included in this study. Patients with distant metastasis, palliative resection, or no information on the preoperative serum CEA levels were excluded. All patients underwent a surgical resection with a laparotomy or laparoscopic surgery. Serum CEA levels were measured in the preoperative period and within 1 month postoperatively. Adjuvant therapy was given to patients with pathologic stage II and III colon cancer. Chemotherapy comprised 5-fluorouracil (5-FU) and leucovorin with or without oxaliplatin. The 1st regimen was to give 5-FU (425 mg/m2) and leucovorin (20 mg/m2) daily for 5 days every 4 weeks for 6 cycles. The 2nd regimen was to give oxaliplatin (85 mg/m2) on the first day and leucovorin (200 mg/m2) before 5-FU injection on days 1 and 2 and 5-FU (400 mg/m2 by bolus and 600 mg/m2 over 22 hours) in days 1 and 2 every 2 weeks for 12 cycles. No adjuvant chemotherapy was administered to patients who refused chemotherapy, were older than 75 years of age, or showed a poor performance status.
Patients were followed up every 3 months for the first 2 years after surgery and every 6 months thereafter for 3 years, for a total 5-years' follow-up. History, physical examination, and serum CEA level were determined at each follow-up visit. Chest X-ray and abdominopelvic computed tomography were performed at 6-month intervals, and colonoscopy was performed annually. Recurrence was identified by imaging studies and colonoscopy and was confirmed by colonoscopic or percutaneous biopsy. When histologic confirmation was not possible, radiologically-observed tumor growth within the previous surgical field was considered to indicate recurrence.
Categorical variables were compared using a chi-square test or a Fisher exact test. The Kaplan-Meier method was conducted to evaluate the OS and the DFS rates. Prognostic factors for OS and DFS were analyzed with a log-rank test, and a multivariate analysis was performed using a Cox proportional hazard model. A P-value of <0.05 was considered to indicate statistical significance. Statistical analyses were conducted using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA).
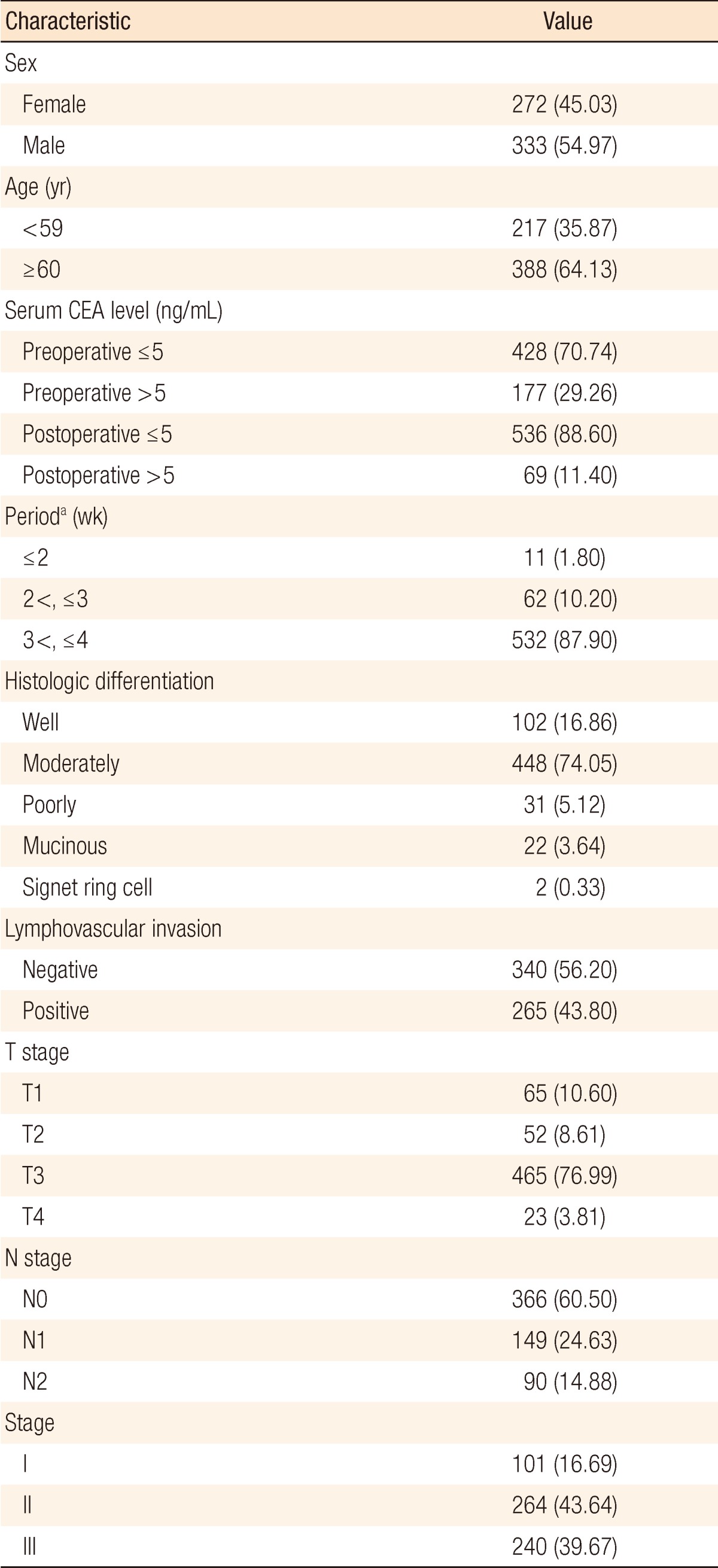
The clinicopathologic characteristics of the colon-cancer patients are shown in Table 1. A high preoperative serum CEA level (>5 ng/mL) was found in 177 of the 605 patients (29.26%). Sixty-nine patients (11.4%) had an abnormal CEA level (>5 ng/mL) after primary resection of the colon cancer. The postoperative serum CEA level was normal (Ōēż5 ng/mL) in 536 patients (88.6%). Postoperative serum CEA level was measured between 3 and 4 weeks after surgical resection in almost all patients (87.9%). The American Joint Committee on Cancer system was used for staging [12] and yielded the following final pathologic tumor stages: stage I, 101 patients (16.69%); stage II, 264 patients (43.64%); and stage III, 240 patients (39.67%) (Table 1). Patients with vascular or lymphatic invasion (P = 0.0003), high grade pT stage (P < 0.0001), high grade pN stage (P < 0.0001) and advanced stage (P < 0.0001) were significantly more associated with increased preoperative CEA levels (Table 2).
To predict the risk of death, we measured the area under the curve of the receiver operating characteristic curve and calculated the cutoff value with maximum sensitivity and specificity in terms of the perioperative rate of decrease in the serum CEA level. The "cutoff values" for the perioperative rate of decrease in the serum CEA level that determined the OS and the DFS were 48.9% and 50.8%, respectively.
In the univariate analysis of a preoperative serum CEA level of >5 ng/mL, prognostic factors for the OS and the DFS were the cutoff value (P < 0.0001), pN stage (P < 0.0001) and stage (P = 0.0172) (Table 3). Meaningful factors in the univariate analysis were included in the multivariate analysis. In the multivariate analysis of a preoperative serum CEA level of >5 ng/mL, prognostic factors for the OS and the DFS were the cut-off value (P < 0.0001) and pN stage (P < 0.0001) (Table 4). Our analyses were based on the following groups:
Group A: preoperative serum CEA of Ōēż5 ng/mL, group B: preoperative serum CEA of >5 ng/mL, group C: preoperative serum CEA of >5 ng/mL and postoperative serum CEA of Ōēż5 ng/mL, group D: preoperative serum CEA of >5 ng/mL and postoperative serum CEA of >5 ng/mL, group E: preoperative serum CEA of >5 ng/mL and below the cutoff value relative to the OS, group F: preoperative serum CEA of >5 ng/mL and in excess of the cutoff value relative to the OS, group G: preoperative serum CEA of >5 ng/mL and below the cutoff value relative to the DFS, group H: preoperative serum CEA of >5 ng/mL and in excess of the cutoff value relative to the DFS.
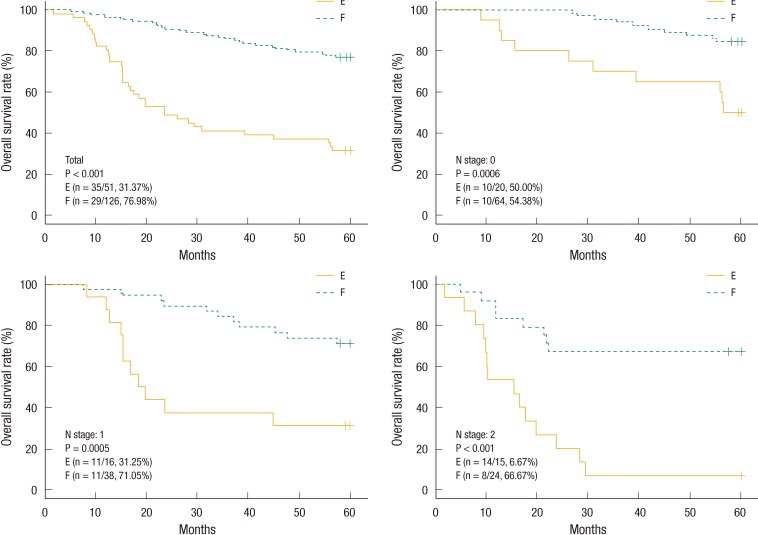
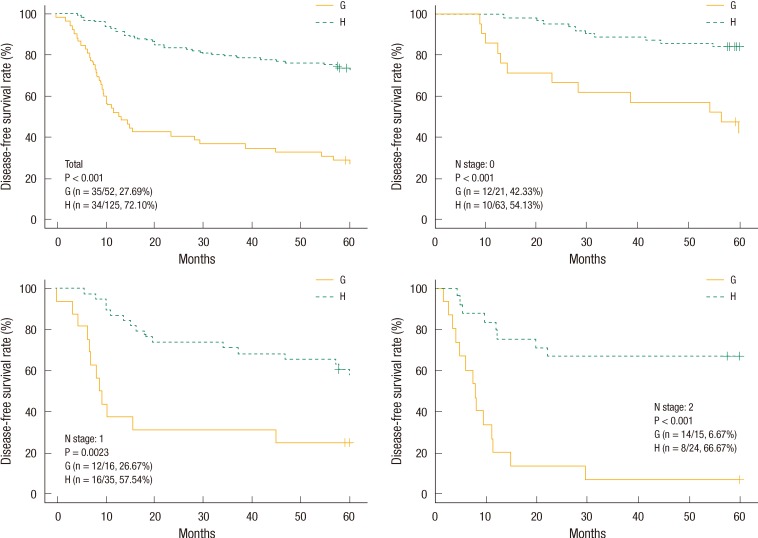
The estimated 5-year OS and DFS rates for group A vs. B were 82.69% vs. 63.84% (P < 0.001) and 78.78% vs. 59.50%, respectively (P < 0.001). The estimated 5-year OS and DFS rates for group C vs. D were 79.37% vs. 25.49% (P < 0.001) and 73.61% vs. 24%, respectively (P < 0.001). The "cutoff values" of the perioperative CEA decrease rates that determined the 5-year OS and DFS rates were 48.9% and 50.8%, respectively. The estimated 5-year OS rates for group E vs. F were 31.37% vs. 76.98%, respectively (P < 0.001) (Fig. 1). The estimated 5-year DFS rates for group G vs. H were 27.69% vs. 72.10%, respectively (P < 0.001) (Fig. 2). We analyzed four groups (groups E-H) for each pN stage. In the analysis of each group for the same pN stage, group E showed a better OS than group F (Fig. 1), and group G showed a better DFS than group H (Fig. 2).
Molecular and biochemical markers of CRC are currently being used to investigate several prognostic factors. However, the studies of these markers are complicated, expensive, and of limited clinical relevance. Therefore, CEA levels, which can be easily determined using a simple blood test before and after surgery, are widely used as prognostic factors in patients with CRC. CEA is the most widely accepted and frequently used tumor marker worldwide in patients with CRC, and the method of measurement is standardized, readily available, and inexpensive. In 1999, the College of American Pathologists Consensus Statement classified the prognostic factors of CRC based on the published evidence. It suggested that preoperative CEA elevation be classified into category 1, together with tumor extent, regional lymph node metastasis, lymphovascular invasion, and residual tumor, after surgery [13]. Most studies of CEA in CRC have focused on the prognostic effect of preoperative CEA levels and on the usefulness of postoperative CEA monitoring for early detection of recurrence after curative surgery and for assessment of the response to chemotherapy in metastatic CRC [14-17].
However, there are few studies of the effect of postoperative serum CEA level on the recurrence rate after a curative resection of colon cancer. A few studies have evaluated the relationship between perioperative serum CEA changes and the prognoses in patients with CRC [18-22]. Park et al. [21] reported that in patients with stage III CRC, the perioperative serum CEA changes were predictive of systemic recurrence and prognosis, and the preoperative and early postoperative serum CEA levels were useful prognostic indicators.
Our data showed that the group with a preoperative serum CEA level of >5 ng/mL had a more advanced stage and more positive lymphovascular invasion than the group with a preoperative serum CEA level of Ōēż5 ng/mL and that the majority of the patients had stages II and III disease. Unlike other studies, this study analyzed association of the rate of decrease in the perioperative serum CEA level and its cutoff value with the survival rate. In the group with a preoperative serum CEA of >5 ng/mL, the cutoff values related to the OS and the DFS were 48.9% and 50.8%, respectively. In the multivariate analysis for the prognostic factors, the cutoff values related to the OS and the DFS showed hazard ratios of 4.951 (95% confidence interval, 2.997 to 8.180) (P < 0.0001) and 4.818 (95% confidence interval, 2.998 to 7.744) (P < 0.0001), respectively. Known prognostic factors for colon cancer were the preoperative serum CEA level, pT stage, pN stage, and lymphovascular invasion. However, in our study the cutoff values and the pN stage were prognostic factors for the OS and the DFS. We think that our results were different from other studies because we analyzed the group with preoperative serum CEA >5 ng/mL, for which a poor prognosis is expected.
When the groups were divided according to a preoperative serum CEA level of 5 ng/mL, the 5-year OS rates for the Ōēż5 and >5 ng/mL groups were 82.69% and 63.84%, respectively (P < 0.0001), and the 5-year DFS rates for the Ōēż5 and >5 ng/mL groups were 78.78% and 59.50%, respectively (P < 0.0001). Therefore, the preoperative serum CEA level was a prognostic factor for survival, as previously shown.
The group with a preoperative serum CEA level of >5 ng/mL was expected to have a poorer outcome. The group with a postoperative serum CEA level that returned to the normal range (group C) showed superior OS and DFS compared with the group with a CEA level that did not return to the normal range. This is similar to the conclusions of Park et al. [21]. Therefore, we suspected that patients with a preoperative serum CEA level of >5 ng/mL would have a good prognosis in terms of the postoperative serum CEA level being reduced compared with preoperative value. We analyzed the "cutoff value" of the perioperative serum CEA decrease rate that determined the OS and the DFS in patients with a preoperative serum CEA level of >5 ng/mL. The 5-year OS rates for patients with a level above the cutoff value and for those with a level below the cutoff value were 76.98% and 31.37%, respectively (P < 0.0001). The 5-year DFS rates for patients with a level above the cutoff value and for those with a level below the cutoff value were 72.10% and 27.69%, respectively (P < 0.0001).
The present study was limited by its retrospective design and by the fact that the time of postoperative blood sampling for the CEA measurement was not consistent, ranging from 1 to 4 weeks after surgery.
In conclusion, normalization of the postoperative serum CEA level and a reduction in excess of 50% in the perioperative rate of decrease in the serum CEA level may be used as a prognostic marker for survival in patients with a preoperatively-elevated CEA level. In addition, if the perioperative rate of decrease in the serum CEA level is not satisfactory, new follow-up strategies will be planned.
References
1. Macdonald JS. Adjuvant therapy of colon cancer. CA Cancer J Clin 1999;49:202ŌĆō219. PMID: 11198882.


2. In: Greene FL, Balch CM, Fleming ID. editors. AJCC cancer staging handbook: TNM classification of malignant tumors. 6th ed. New York: Springer; 2002.
3. Wang JY, Lu CY, Chu KS, Ma CJ, Wu DC, Tsai HL, et al. Prognostic significance of pre- and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. Eur Surg Res 2007;39:245ŌĆō250. PMID: 17457032.


4. Klee GG, Go VJW. In: Ghosh BC, Ghosh L,Carcinoembryonic antigen and its role in clinical practice. editors. Tumor markers and tumor-associated antigens. New York: Mc-Graw-Hill; 1990. p. 22ŌĆō43.
5. Arfa N, Hamdani I, Gharbi L, Ben Abid S, Ghariani B, Mannai S, et al. Survival and prognostic factors of colorectal adenocarcinoma: analytic multifactor review of 150 cases. Ann Chir 2006;131:104ŌĆō111. PMID: 16443189.


6. Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, et al. Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol 2000;30:12ŌĆō16. PMID: 10770562.


7. Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965;122:467ŌĆō481. PMID: 4953873.



8. Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC, Bunn PA Jr, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res 1998;58:1149ŌĆō1158. PMID: 9515799.

9. Chen CC, Yang SH, Lin JK, Lin TC, Chen WS, Jiang JK, et al. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res 2005;124:169ŌĆō174. PMID: 15820244.


10. Moertel CG, O'Fallon JR, Go VL, O'Connell MJ, Thynne GS. The preoperative carcinoembryonic antigen test in the diagnosis, staging, and prognosis of colorectal cancer. Cancer 1986;58:603ŌĆō610. PMID: 3731019.


11. Steele G Jr, Osteen RT, Wilson RE, Brooks DC, Mayer RJ, Zamcheck N, et al. Patterns of failure after surgical cure of large liver tumors. A change in the proximate cause of death and a need for effective systemic adjuvant therapy. Am J Surg 1984;147:554ŌĆō559. PMID: 6324604.


12. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
13. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979ŌĆō994. PMID: 10888773.

14. Quentmeier A, Schlag P, Hohenberger P, Schwarz V, Abel U. Assessment of serial carcinoembryonic antigen: determinations to monitor the therapeutic progress and prognosis of metastatic liver disease treated by regional chemotherapy. J Surg Oncol 1989;40:112ŌĆō118. PMID: 2915539.


15. Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg 1994;219:174ŌĆō182. PMID: 8129488.



16. Sugarbaker PH, Gianola FJ, Dwyer A, Neuman NR. A simplified plan for follow-up of patients with colon and rectal cancer supported by prospective studies of laboratory and radiologic test results. Surgery 1987;102:79ŌĆō87. PMID: 3589978.

17. Tate H. Plasma CEA in the post-surgical monitoring of colorectal carcinoma. Br J Cancer 1982;46:323ŌĆō330. PMID: 7126423.



18. Slentz K, Senagore A, Hibbert J, Mazier WP, Talbott TM. Can preoperative and postoperative CEA predict survival after colon cancer resection? Am Surg 1994;60:528ŌĆō531. PMID: 8010568.

19. Oh JH, MacLean LD. Prognostic use of preoperative and immediate postoperative carcinoembryonic antigen determinations in colonic cancer. Can J Surg 1977;20:64ŌĆō67. PMID: 832207.

20. Behr TM, Sharkey RM, Juweid MI, Dunn RM, Ying Z, Zhang CH, et al. Factors influencing the pharmacokinetics, dosimetry, and diagnostic accuracy of radioimmunodetection and radioimmunotherapy of carcinoembryonic antigen-expressing tumors. Cancer Res 1996;56:1805ŌĆō1816. PMID: 8620497.

21. Park YA, Lee KY, Kim NK, Baik SH, Sohn SK, Cho CW. Prognostic effect of perioperative change of serum carcinoembryonic antigen level: a useful tool for detection of systemic recurrence in rectal cancer. Ann Surg Oncol 2006;13:645ŌĆō650. PMID: 16538413.


22. Lee WS, Baek JH, Kim KK, Park YH. The prognostic significant of percentage drop in serum CEA post curative resection for colon cancer. Surg Oncol 2012;21:45ŌĆō51. PMID: 21094039.