- Search
| Ann Coloproctol > Volume 37(4); 2021 > Article |
|
Abstract
Purpose
The survival benefit of neoadjuvant chemotherapy (NAC) prior to surgical resection in colorectal cancer with liver metastases (CRCLM) patients remains controversial. The aim of this study was to compare overall outcome of CRCLM patients who underwent NAC followed by surgical resection versus surgical treatment first.
Methods
We retrospectively analyzed 429 patients with stage IV colorectal cancer with synchronous liver metastases who underwent simultaneous liver resection between January 2008 and December 2016. Using propensity score matching, overall outcome between 60 patients who underwent NAC before surgical treatment and 60 patients who underwent surgical treatment first was compared.
Results
Before propensity score matching, metastatic cancer tended to involve a larger number of liver segments and the primary tumor size was bigger in the NAC group than in the primary resection group, so that a larger percentage of patients in the NAC group underwent major hepatectomy (P < 0.001). After propensity score matching, demographic features and pathologic outcomes showed no significant differences between the 2 groups. In addition, there was no significant difference in short-term recovery outcomes such as postoperative morbidity (P = 0.603) and oncologic outcome, including 3-year overall survival rate (P = 0.285) and disease-free survival rate (P = 0.730), between the 2 groups.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide and the second most common cause of cancer death [1]. In total, 20% to 25% of CRC patients have stage IV disease, 15% to 25% of whom have synchronous CRC with liver metastases (CRCLM) [2]. Untreated CRCLM shows a very poor prognosis; 1- and 5-year survival rates of untreated CRCLM are less than 30% and 5%, respectively [2]. Surgical resection is the only treatment, which significantly increases the long-term survival of patients with CRCLM [2].
However, there is no definite treatment guideline for CRCLM since there are no randomized studies assessing the outcome of surgical resection compared to other treatment options such as systemic chemotherapy and localized treatment. In addition, many CRCLM patients have unresectable liver metastases, so that surgical resection may not be the first choice. Moreover, even if liver metastases are resectable, relapse is common after surgical resection [3].
Therefore, neoadjuvant chemotherapy (NAC) can be considered prior to surgical resection for potentially resectable CRCLM for several reasons; reducing the size of primary cancer, increasing the possibility of curative resection of liver metastases, and improving progression-free survival [4].
In this study, we will discuss the outcomes of resectable CRCLM patients who underwent NAC followed by surgical resection compared to those who received surgical treatment first.
We collected patient data from the prospectively managed CRC database at Samsung Medical Center in Seoul, Korea. Insufficient data were reevaluated using electronic medical charts. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (No. 2020-08-069). Written informed consent was waived by the IRB. We reviewed 444 patients with resectable stage IV CRC with synchronous liver metastases who underwent simultaneous liver resection between January 2008 and December 2016. The initial resectability of liver metastases at the time of diagnosis was evaluated by the same hepatobiliary team. Among 444 patients, 15 patients who received more than 8 cycles of NAC were excluded as they were considered impossible to treat surgically at the time of diagnosis. Therefore, only 66 CRCLM patients who were determined to have resectable liver metastasis were included in NAC group and 363 underwent resection of CRC and liver metastases first.
Patients who had metastasis in organs other than liver or bowel complications including obstruction and perforation were excluded in this study. Patients who received palliative chemotherapy were also excluded from this study.
All patients underwent preoperative staging work-up with chest and abdominopelvic computed tomography (CT). Liver magnetic resonance imaging (MRI) was also performed to further assess the extent of liver metastases. Whole-body positron emission tomography (PET)-CT scanning was performed selectively for some patients to determine the presence of distant metastases besides liver. The treatment plan for each patient was determined by a multidisciplinary team consisting of surgeons, oncologists, radiologists, and other related specialists.
During the follow-up period, patients visited the outpatient clinic every 3 months for the first 2 years, every 6 months for the subsequent 3 years, and annually thereafter. We performed physical examinations on the patients and checked their regular laboratory tests, including serum carcinoembryonic antigen (CEA), every 3 months. Chest CT and abdominopelvic CT scans were performed every 6 months or every year during the follow-up period. When recurrence was suspected, CT scans, MRI, and PET scans were performed.
Differences between the 2 groups were compared using the chi-square test, Fisher exact test, or the Mann-Whitney U-test as appropriate. Propensity score adjustment was performed to reduce the effect of selection biases that can influence the choice of approach and oncologic outcomes, such as age, sex, body mass index (BMI), American Society of Anesthesiologists physical status classification, postoperative chemotherapy, surgical procedures (minor and major liver resections, rectal resection), number of liver segments involving metastatic lesions, chemotherapy regimen, and target agent use status. After propensity score matching, perioperative data, postoperative mortality and morbidity, and oncologic outcomes were compared between the preoperative group and primary resection group. Survival rates were calculated by the Kaplan-Meier method. Survival curves were compared using the log-rank test. A P-value of < 0.05 was considered statistically significant for all results. All statistical analyses were performed using IBM SPSS Statistics for Windows, ver. 23.0 (IBM Corp., Armonk, NY, USA).
Demographic and pathologic data for all 429 patients are summarized in Table 1. Among 429 patients with CRC and synchronous colorectal liver metastasis, 66 patients underwent resection after NAC, and 363 patients underwent primary resection without NAC. There were no significant differences in sex, BMI, or size of liver metastatic lesion between the 2 groups. The median ages of the NAC and primary resection groups were 54 ┬▒ 11 years and 60 ┬▒ 12 years, respectively (P < 0.001). The mean diameters of primary tumors in the NAC and primary resection groups were 3.9 ┬▒ 2.0 cm and 5.4 ┬▒ 2.0 cm, respectively (P < 0.001).
There were significant differences between the 2 groups for pathological (p) T category of primary cancer. pT3/4 tumors were significantly more common in the primary resection group than in the NAC group (84.8% vs. 95.6%, P = 0.001), whereas pT1/2 tumors are more common in NAC group (15.2% vs. 4.4%, P = 0.001).
Metastatic cancer involving only 1 liver segment was 15 (22.7%) in the NAC group and 199 (54.8%) in the primary resection group, whereas metastatic cancer involving more than 4 liver segments was 21 (31.8%) in the NAC group and 33 (9.1%) in the primary resection group (P < 0.001). After propensity score matching, demographic features and pathologic outcomes showed no significant differences between the 2 groups.
Among 66 patients who underwent NAC, there was a median of 5 cycles of NAC, and 37 (56.1%) patients received no more than 5 cycles of NAC. The median interval between NAC and surgical treatment was 32 ┬▒ 12 days (Table 2). Postoperative adjuvant chemotherapy was administered to 58 (87.9%) patients in the NAC group and 319 (87.9%) patients in the primary resection group (P > 0.999). Unlike NAC, oxaliplatin-based chemotherapy was the most common postoperative chemotherapeutic treatment regimen in both groups (P < 0.001). Target agents were administered to 22 (33.3%) patients in the NAC group and 37 (10.2%) patients in the primary resection group (P < 0.001). After propensity score matching, there were no significant differences between the 2 groups.
Types of surgical procedures for simultaneous resection of CRC and synchronous liver metastases are detailed in Table 3. Major hepatectomy was performed in 54.5% and 31.4% of patients in the NAC and primary resection groups, respectively (P < 0.001). Minor hepatectomy was performed in 45.5% and 68.6% of patients in the NAC and primary resection groups, respectively (P < 0.001). Rectal resection was performed in 83.3% and 70.3% of patients in the NAC and primary resection groups, respectively (P = 0.042). After propensity score matching, there were no significant differences in operating procedure between the 2 groups.
Perioperative outcomes of the NAC and primary resection groups are shown in Table 4. Only operation time was significantly longer in the NAC group than in the primary resection group before propensity score matching (336 minutes vs. 297 minutes, P = 0.005). After propensity score matching, the 2 groups did not show a significant difference in total operation time (333 minutes vs. 323 minutes, P = 0.591), transfusion rate (14.1% vs. 5.6%, P = 0.091), time to soft diet (2.9 days vs. 3.0 days, P = 0.633), or length of hospital stay (12 days vs. 14 days, P = 0.443). Also, there was no significant difference in overall postoperative morbidity between the 2 groups (25% vs. 28%, P = 0.603). There were 2 postoperative mortalities in the primary resection group due to the following reasons: (1) bowel ischemia and postoperative intraabdominal bleeding and (2) septic shock with anastomosis site leakage.
Short-term recovery outcomes were compared among patients in the NAC group according to the number of cycles of NAC by dividing them into 2 groups (Table 5): one with no more than 4 cycles of NAC and the other with more than 5 cycles of NAC. There was no significant difference between the 2 groups.
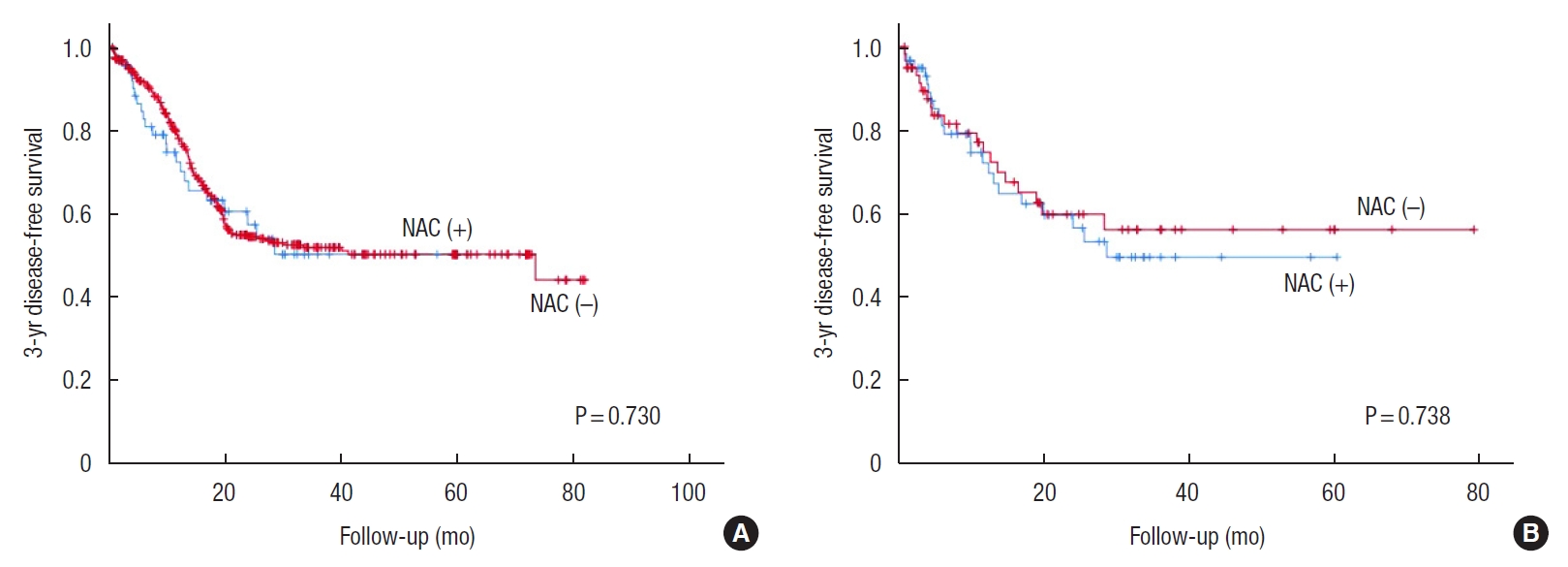
Oncologic outcomes for matched patients are shown in Figs. 1, 2. There were no differences in 3-year overall survival rate between the NAC and primary resection groups before and after propensity score matching (before propensity score matching: 81.7% vs. 79.0%, P = 0.613; after propensity score matching: 81.0% vs. 67.9%, P = 0.285). Postoperative 3-year disease-free survival rate was also not better in the NAC group than in the primary resection group before and after propensity score matching (before propensity score matching: 49.7% vs. 51.4%, P = 0.730; after propensity score matching: 49.1% vs. 55.7%, P = 0.738).
Although surgical resection is the standard treatment for CRCLM, only 15% to 20% of CRCLM patients present with resectable hepatic metastases at the time of the diagnosis [5]. Moreover, 5-year survival of resectable CRCLM patients who receive surgical treatment reaches only 30% to 60% [6, 7]. Advances in surgical techniques, improved chemotherapy, and multidisciplinary discussions have recently made it possible to improve resectability and survival of CRCLM [8].
The concept of resectability has changed over recent decades. Currently, CRCLM is defined as resectable when the following conditions are met: (1) the metastatic lesion can be resected completely with a negative margin, (2) 2 adjacent liver segments with intact vascular inflow and outflow as well as biliary drainage can be preserved after resection, and (3) sufficient future liver remnant after resection [9, 10]. However, there is no consensus for CRCLM resectability criteria due to lack of randomized trials, advent of novel techniques such as ablative therapy, and portal vein embolization, among others. Some studies suggest risk-scoring systems for patient selection [11], but most multidisciplinary teams depend on clinical experience and local surgical expertise rather than those scoring systems [12].
Two multicenter randomized phase III trials have been performed to evaluate the benefit of adjuvant chemotherapy after surgical treatment of CRCLM patients. However, both trials were terminated early and failed to show a statistically significant difference in overall survival because of slow accrual [13, 14]. According to the European Organisation for Research and Treatment of Cancer (EORTC) Intergroup trial 40983 by Nordlinger et al. [15], perioperative FOLFOX4 (fluorouracil, leucovorin, oxaliplatin) chemotherapy decreased the recurrence rate by a quarter and was compatible with major surgery in patients with resectable CRCLM. Nordlinger et al. [16] continued analysis to compare the long-term overall survival in patients who underwent perioperative chemotherapy with those who underwent surgery alone, but the study could not demonstrate a statistically significant difference in overall survival between the 2 groups. Also, whether NAC can improve the overall outcome of CRCLM patients is still controversial.
One previous study demonstrated that NAC in patients with CRCLM did not improve overall survival or surgical resectability [17]. Therefore, they insisted that CRCLM patients should not routinely undergo NAC if the metastatic tumor is resectable. In another study, not all patients with resectable CRCLM received a survival benefit from NAC, but they found 4 independent prognostic factors (e.g., a primary tumor at stage T4, Ōēź 4 liver metastases, the size of metastatic tumor of Ōēź 5 cm in diameter, and a serum CEA level of Ōēź 5 ng/mL) and showed that resectable CRCLM patients with more than 2 of those risk factors received a survival benefit from NAC [18].
In this study, short-term recovery and oncologic outcome of all CRCLM patients were compared between those who received NAC and those who did not use propensity score matching analysis. More liver segments with multiple metastatic lesions were involved in the NAC group and tended to receive major hepatectomy. This shows that patients in the NAC group were likely to present with more advanced metastases. These might rather underestimate the oncologic benefit of NAC. It is estimated that NAC made the lesions possible to be treated well by down-staging or down-sizing the lesions because the size of the primary tumor had decreased after 8 cycles of chemotherapy. Nevertheless, postoperative morbidity after surgical treatment in the NAC group was not inferior to that of the primary resection group, which means that at least NAC does not increase postoperative complications. In addition, oncologic outcomes such as overall survival and disease-free survival were not different between the 2 groups and after propensity score matching.
There are several limitations to this study. This was a retrospective study with a single-center design. Although we performed propensity score matching to minimize bias between the 2 groups, inevitable selection bias still remained. Due to the lack of universal consensus for CRCLM resectability criteria, it was hard to evaluate resectability throughout patients in the neoadjuvant group. In addition, patients in the NAC group had the relatively more advanced disease in general and many hemato-oncologists have recently tended to treat these patients with NAC prior to surgical treatment.
Although it is based on retrospective single-center data, this study is meaningful because a considerably large number of patients were analyzed using propensity score matching analysis. The criteria of liver resectability for CRCLM in our institution mostly follow a general definition. If it is technically possible and the function of remnant liver after resection is sufficient, surgical resection is performed. Since we fully acknowledged that resectability can be affected by a physicianŌĆÖs technical skills, only the cases discussed by the multidisciplinary team involving the same hepatobiliary team were included in this study to minimize selection bias. Patients treated with more than 8 cycles of chemotherapy were excluded in order to exclude initially unresectable cases that converted to resectable cases due to NAC.
In our institution, chemotherapy is the first treatment of choice for locally advanced rectal cancer patients with distant metastasis. For those patients with resectable liver metastasis, short-term radiotherapy is mostly performed before or after NAC, and the usual regimen of radiotherapy can be changed in these cases. Therefore, we decided to exclude those cases in order to eliminate the biased outcome that may result from radiotherapy.
In conclusion, NAC did not significantly decrease short-term recovery and oncologic outcomes in advanced CRCLM patients. From these results, we carefully concluded that NAC before surgical treatment can be considered in advanced resectable CRCLM patients.
For further evaluation, we need universal consensus for resectability criteria of CRCLM and require study of the prognosis of NAC on CRCLM patients before surgical resection according to their resectability. In addition, randomized controlled trials are necessary to reduce selection bias, although they will be difficult due to ethical issues.
Fig.┬Ā1.
Three-year overall survival curves for the neoadjuvant chemotherapy (NAC) (+) and NAC (ŌĆō) groups (A) before matching and (B) after matching.

Fig.┬Ā2.
Three-year disease-free survival curves for the neoadjuvant chemotherapy (NAC) (+) and NAC (ŌĆō) groups (A) before matching and (B) after matching.
Table┬Ā1.
Demographic and pathologic data before and after propensity score matching
| Variable |
Before propensity score matching |
After propensity score matching |
||||
|---|---|---|---|---|---|---|
| NAC (+) (n=66) | NAC (ŌĆō) (n=363) | P-value | NAC (+) (n=60) | NAC (ŌĆō) (n=60) | P-value | |
| Age (yr) | 54 ┬▒ 11 | 60 ┬▒ 12 | < 0.001 | 53 ┬▒ 11 | 56 ┬▒ 11 | 0.098 |
| Body mass index (kg/m2) | 23.2 ┬▒ 2.8 | 23.4 ┬▒ 2.9 | 0.519 | 23.2 ┬▒ 2.8 | 23.1 ┬▒ 2.7 | |
| Sex | 0.489 | 0.752 | ||||
| ŌĆāMale | 37 (56.1) | 220 (60.6) | 31 (51.7) | 35 (58.3) | ||
| ŌĆāFemale | 29 (43.9) | 143 (39.4) | 29 (48.3) | 25 (41.7) | ||
| Preoperative CEA (ng/mL) | 0.607 | 0.854 | ||||
| ŌĆā<5 | 35 (53.0) | 180 (49.6) | 34 (56.7) | 33 (55.0) | ||
| ŌĆāŌēź5 | 31 (47.0) | 183 (50.4) | 26 (43.3) | 27 (45.0) | ||
| ASA PS classification | 0.691 | 0.697 | ||||
| ŌĆāI, II | 62 (93.9) | 336 (92.6) | 56 (93.3) | 57 (95.0) | ||
| ŌĆāIII, IV | 4 (6.1) | 27 (7.4) | 4 (6.7) | 3 (5.0) | ||
| Pathology T stage | 0.001 | 0.762 | ||||
| ŌĆāT1/T2 | 10 (15.2) | 16 (4.4) | 7 (11.7) | 5 (8.3) | ||
| ŌĆāT3/T4 | 56 (84.8) | 347 (95.6) | 53 (88.3) | 55 (91.7) | ||
| Pathology N stage | 0.749 | 0.452 | ||||
| ŌĆāN0 | 12 (18.2) | 73 (20.2) | 9 (15.0) | 13 (21.7) | ||
| ŌĆāN1 | 29 (43.9) | 137 (37.7) | 26 (43.3) | 20 (33.3) | ||
| ŌĆāN2 | 25 (37.9) | 153 (42.1) | 25 (41.7) | 27 (45.0) | ||
| Segmental involvement | < 0.001 | 0.539 | ||||
| ŌĆā1 | 15 (22.7) | 199 (54.8) | 15 (25.0) | 22 (36.7) | ||
| ŌĆā2 | 21 (31.8) | 107 (29.5) | 20 (33.3) | 15 (25.0) | ||
| ŌĆā3 | 9 (13.6) | 24 (6.6) | 8 (13.3) | 8 (13.3) | ||
| ŌĆāŌēź4 | 21 (31.8) | 33 (9.1) | 17 (28.3) | 15 (25.0) | ||
| Location of primary tumor | 0.225 | 0.367 | ||||
| ŌĆāRight colon | 10 (15.2) | 89 (24.5) | 8 (13.3) | 14 (23.3) | ||
| ŌĆāLeft colon | 35 (53.0) | 180 (49.6) | 33 (55.0) | 29 (48.3) | ||
| ŌĆāRectum | 21 (31.8) | 94 (25.9) | 19 (31.7) | 17 (28.4) | ||
| Size of primary tumor (cm) | 3.9 ┬▒ 2.0 | 5.4 ┬▒ 2.0 | < 0.001 | 4.0 ┬▒ 2.0 | 4.3 ┬▒ 2.1 | 0.660 |
| Liver metastatic lesion sizea (cm) | 2.6 ┬▒ 1.9 | 2.5 ┬▒ 1.2 | 0.434 | 2.7 ┬▒ 2.0 | 2.4 ┬▒ 1.2 | 0.335 |
Table┬Ā2.
Neoadjuvant and adjuvant chemotherapy data before and after propensity score matching
Values are presented as number (%) or mean┬▒standard deviation if otherwise specified.
NAC, neoadjuvant chemotherapy; NA, not applicable; FOLFOX, fluorouracil, leucovorin, oxaliplatin; FOLFIRI, fluorouracil, leucovorin, irinotecan; XELOX, capecitabine, oxaliplatin; XELIRI, capecitabine, irinotecan; MSI, microsatellite instability; MSS, microsatellite stable; MSI-H, MSI high; MSI-L, MSI low.
Target agent: bevacizumab or cetuximab.
Table┬Ā3.
Operating procedure before and after propensity score matching
| Variable |
Before propensity score matching |
After propensity score matching |
||||
|---|---|---|---|---|---|---|
| NAC (+) (n=66) | NAC (ŌĆō) (n=363) | P-value | NAC (+) (n=60) | NAC (ŌĆō) (n=60) | P-value | |
| Hepatectomy | < 0.001 | 0.715 | ||||
| ŌĆāMajora | 36 (54.5) | 114 (31.4) | 32 (53.3) | 30 (50.0) | ||
| ŌĆāMinorb | 30 (45.5) | 249 (68.6) | 28 (46.7) | 30 (50.0) | ||
| Primary tumor resection | 0.042 | 0.307 | ||||
| ŌĆāRHC | 11 (16.7) | 88 (24.2) | 9 (15.0) | 11 (18.3) | ||
| ŌĆāLHC | 0 (0) | 20 (5.5) | 0 (0) | 2 (3.3) | ||
| ŌĆāAR/LAR/uLAR/ISR | 55 (83.3) | 255 (70.3) | 51 (85.0) | 47 (78.4) | ||
| Surgical approach | 0.121 | 0.850 | ||||
| ŌĆāOpen | 42 (63.6) | 265 (73.0) | 38 (63.3) | 37 (61.7) | ||
| ŌĆāMIS | 24 (36.4) | 98 (27.0) | 22 (36.7) | 23 (38.3) | ||
| Stoma formation | 13 (19.7) | 44 (12.1) | 0.095 | 13 (21.7) | 6 (10.0) | 0.080 |
Table┬Ā4.
Comparative short-term recovery outcomes between the neoadjuvant group and primary resection group before and after matched groups
| Variable |
Before propensity score matching |
After propensity score matching |
||||
|---|---|---|---|---|---|---|
| NAC (+) (n=66) | NAC (ŌĆō) (n=363) | P-value | NAC (+) (n=60) | NAC (ŌĆō) (n=60) | P-value | |
| Operation time (min) | 336 ┬▒ 112 | 297 ┬▒ 103 | 0.005 | 334 ┬▒ 112 | 312 ┬▒ 120 | 0.304 |
| Transfusion | 10 (15.2) | 49 (13.5) | 0.720 | 9 (15.0) | 10 (16.7) | 0.803 |
| Time to soft diet (day) | 2.9 ┬▒ 1.4 | 3.0 ┬▒ 1.2 | 0.852 | 2.8 ┬▒ 1.2 | 2.8 ┬▒ 1.0 | 0.869 |
| Length of hospital stay (day) | 14┬▒8 | 14┬▒8 | 0.993 | 14┬▒9 | 13┬▒7 | 0.687 |
| Postoperative mortality (< POD 30)a | 0 (0) | 2 (0.6) | 0.546 | 0 (0) | 0 (0) | > 0.999 |
| Postoperative morbidity | 24 (36.4) | 132 (36.4) | > 0.999 | 22 (36.7) | 23 (38.3) | 0.850 |
| ŌĆāBile leakage | 2 (3.0) | 9 (2.5) | 0.680 | 2 (3.3) | 4 (6.7) | 0.402 |
| ŌĆāSurgical site infection | 9 (13.6) | 49 (13.5) | 0.976 | 8 (13.3) | 6 (10.0) | 0.570 |
| ŌĆāIleus | 7 (10.6) | 37 (10.2) | 0.919 | 5 (8.3) | 4 (6.7) | 0.729 |
| ŌĆāAnastomotic leakage | 2 (3.0) | 19 (5.2) | 0.755 | 2 (3.3) | 3 (5.0) | 0.648 |
| ŌĆāIntraabdominal fluid collection | 4 (6.1) | 21 (5.8) | 0.930 | 4 (6.7) | 4 (6.7) | > 0.999 |
| Intraabdominal bleeding | 1 (1.5) | 5 (1.4) | 0.930 | 1 (1.7) | 2 (3.3) | 0.559 |
Table┬Ā5.
Comparative short-term recovery outcomes according to the cycles of NAC
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394ŌĆō424.


2. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254ŌĆō9.



3. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Fran├¦aise de Chirurgie. Cancer 1996;77:1254ŌĆō62.


4. Nasti G, Ottaiano A, Berretta M, Delrio P, Izzo F, Cassata A, et al. Pre-operative chemotherapy for colorectal cancer liver metastases: an update of recent clinical trials. Cancer Chemother Pharmacol 2010;66:209ŌĆō18.


5. Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509ŌĆō20.



6. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759ŌĆō66.



7. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995;19:59ŌĆō71.


8. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677ŌĆō83.



9. Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1261ŌĆō8.


10. Rocha FG, Helton WS. Resectability of colorectal liver metastases: an evolving definition. HPB (Oxford) 2012;14:283ŌĆō4.



11. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309ŌĆō18.



12. Khan K, Wale A, Brown G, Chau I. Colorectal cancer with liver metastases: neoadjuvant chemotherapy, surgical resection first or palliation alone? World J Gastroenterol 2014;20:12391ŌĆō406.



13. Langer B, Bleiberg H, Labianca R, Shepherd L, Nitti D, Marsoni S, et al. Fluorouracil (FU) plus l-leucovorin (l-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial. Proc Am Soc Clin Oncol 2002;21:149a.
14. Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24:4976ŌĆō82.


15. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007ŌĆō16.



16. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208ŌĆō15.


-
METRICS

- Related articles in ACP
-
Timing of Adjuvant Chemotherapy in Colorectal Cancer Patients2013 August;29(4)







