- Search
| Ann Coloproctol > Epub ahead of print |
|
Abstract
Purpose
Retrorectal tumors (RTs) are rare tumors that arise in the space between the mesorectum and the pelvic wall and often originate in embryonic tissues. The primary treatment for these tumors is complete excision surgery, and choosing the best surgical approach is very important.
Methods
In this study, we retrospectively collected the data of 15 patients with RTs who underwent surgery in Imam Khomeini Hospital (Tehran, Iran) for 12 years to share our experiences of patients’ treatment and compare different surgical approaches.
Results
A total of 5 tumors were malignant, 10 were benign, and most of the tumors were congenital. Malignant tumors were seen in older patients. Three surgical procedures were performed on patients. Three patients underwent abdominal approach surgery, and 8 patients underwent posterior surgery. A combined surgical approach was performed on 4 patients. Two patients underwent laparoscopic surgery. The abdominal approach had the least long-term complication, and the combined approach had the most complications; laparoscopic surgery reduced the length of hospital stay and complications after surgery.
Conclusion
A multidisciplinary team collaboration using magnetic resonance imaging details is necessary to determine a surgical treatment approach. It could reduce the need for a preoperative biopsy. However, every approach has its advantages and disadvantages, and individualized treatment is the key.
Retrorectal tumors (RTs) or presacral tumors are sporadic tumors forming between the mesorectum and the pelvic wall. These tumors are often misdiagnosed due to vague and mild clinical symptoms; therefore, their prevalence is not well known. The incidence of these tumors is 1 in every 40,000 hospitalizations in the Mayo Clinic and is more common in women than men [1, 2]. Benign tumors are usually cystic, and malignant are usually solid tumors that contain necrotic areas and tend to invade surrounding tissues. Although most tumors in this area are benign, malignant tumors are not uncommon [3, 4]. Pathologically, these tumors vary depending on the location of the fetal origin. In this base, RTs, in order of prevalence, are congenital, neurogenic, osseous, inflammatory, and miscellaneous, and each of these groups is divided into more complex subgroups [5].
In the tumor diagnosis process, imaging is performed before surgery in almost all cases. Imaging, especially magnetic resonance imaging (MRI), is helpful for surgical planning [4]. Biopsy is not recommended due to complications and diagnostic errors, and this issue increases the importance of preoperative imaging and perfect surgery [6, 7]. However, some new studies suggest that tumor biopsy could be safe and beneficial for treatment planning [3].
The approach to these tumors depends on various factors, including the tumor’s location and size and the malignancy’s presence. The basis of treatment for these tumors is surgery. Even benign-looking tumors in this area require complete resection because sometimes they have malignant components, or have the potential to become malignant [8]. Without accurate surgery, the patient will experience recurrence and repeated surgeries. We know that benign tumors in this area can cause urinary tract and meningeal infections [7]. Surgery is still the main treatment in RTs due to the ineffectiveness of other treatments such as chemotherapy and radiation therapy. Primary RTs are resistant to chemotherapy, and radiotherapy is used only for palliation in these patients [9].
The manifestations, diagnosis, and treatment of RTs can confuse the physician; surgery can be complicated because of the tumor location and the area’s anatomy. On the other hand, surgery is the most important part of treating these patients, and choosing the appropriate surgical approach can increase the success of tumor resection and reduce injuries and complications. In presacral tumors, the prognosis of patients is directly related to complete tumor resection [9, 10].
The surgical approach for these tumors varies depending on the location and nature of the tumor. Classically, tumors that do not extend below the S3 vertebrae can be removed through the abdomen (anterior approach), lower tumors through the sacral (posterior or perineal approach), and tumors that are palpable on the rectal examination can be removed through the rectum. Larger or middle position tumors may need to be operated on by combining the abdominal and sacral approaches. In presacral tumors, a rectal invasion requires removal of the rectum, and a coccyx invasion requires coccygectomy or sacrectomy. Complex cases require a multidisciplinary team of the colon or rectal surgeons, orthopedists, neurosurgeons, and plastic surgeons [11, 12].
Although experience with minimally invasive surgery for RTs is limited, laparoscopic and endoscopic transrectal microsurgery have been reported. Minimally invasive surgery reduced the length of hospital stay but did not affect reducing surgical complications. It reduces pain and recovery time after surgery and is cosmetically better and has been shown to have no less surgical and oncological outcomes than transabdominal surgery [8, 13].
In RT surgeries, important proximities of this area, such as the rectum and dura, increase the importance of accurate surgery in this area. In other words, surgery should be performed with complete resection while taking care of the essential regions, if possible, which shows the importance of a proper surgical approach in tumors of this area. The prognosis of these tumors after surgery is excellent if they are benign and between 43% and 75% if malignant [14].
Despite the advances in surgery in recent decades, the treatment of RTs is still challenging; the resistance of these tumors to radiochemotherapy indicates the need for accurate surgical guidelines as the primary treatment for these patients. Due to the lack of evidence in the treatment and surgical approaches of RTs, we conducted this study to review epidemiology and pathology, some other properties of RTs treated and undergoing excision at our hospital, and experience of our approaches for these tumors and surgical details, with patient’s outcomes in follow-up.
This study was approved by the Institutional Research Ethics Committee of Imam Khomeini Hospital Complex, Tehran University of Medical Sciences (No. IR.TUMS.IKHC.REC.1399.141). Informed consent was obtained with respecting the confidentiality of patients.
Medical records of 15 patients who underwent surgery for RTs at the general surgery ward in Tehran University of Medical Science (Tehran, Iran) between 2009 and 2021 were reviewed retrospectively. Patients under 18 years old and who have tumors with invasion of adjacent organs to retrorectal space were excluded; the patients’ demographic characteristics, signs and symptoms, physical examination, imaging, surgical information, tumor pathology, and 6-month patient follow-up were documented. All patients underwent a radiological examination before surgery; imaging (MRI or computed tomography [CT] scan) findings were reported by a radiologist. Imaging report items including tumor size and location, invasion to neighbor structures, and tumor morphology were collected.
Three major surgical approaches depending on tumor features in imaging were used to remove RTs. The anterior (abdominal) approach is usually for patients with RTs above S3, the posterior approach (transsacral or transperineal) usually for patients with RTs below S3, the combined anterior and posterior approach for large tumors or tumors above and below S3; and laparoscopic surgery (anterior approach) for exceptional cases.
The posterior approach (Fig. 1) started with the patient in a prone jackknife position; for smaller lesions, we made a longitudinal incision posterior to the sacrum, and the coccyx and lower sacral bones were resected to gain access for resection of the tumor. But in larger or invasive tumors in which we usually had bone involvement, to provide maximal exposure and identification of the sacral roots, the proximal part of the sacral bone uninvolved by the tumor was resected to reach the normal presacral tissue, and the rectum; from there we usually tried to dissect the tumor from the rectum. We performed this proximal to distal approach to preserve the rectum and early identification of the sacral roots.
In the abdominal approach (Fig. 2), the patient was in the lithotomy position. When dissecting the rectum, we started and continued in the total mesorectal excision plane to reduce bleeding and have the best exposure to the lesion. While dissecting the lesion’s posterior surface, we tried to avoid elevating the fascia to prevent fatal bleeding, and gentle separation of the posterior part with peanut was done. A combined approach (Fig. 3) started with a laparotomy and dissection of the rectum, then the abdomen has closed, and the patient was placed in a prone position; for the posterior approach, a combined approach was usually used for large cysts or masses below and under the levator muscles, for supralevator mass with lengthy perineal extension (dumbbell-shaped lesion), and supralevator lesions with sacral involvement.
Tumor and lymph nodes and other resected tissues were examined for pathology, and factors such as tumor type, presence of malignancy, free resection margins, and malignancy in lymph nodes were reported. Patients were followed up for 6 months after surgery with a history and examination in a clinic or telephone interview, and MRI was performed 6 months after surgery. Patients were evaluated for evidence of tumor recurrence and mortality if it happened.
Statistical analyses were performed with IBM SPSS ver. 24 (IBM Corp). The Mann-Whitney test was used to compare the age of patients and the size of tumors between the 2 groups with benign and malignant tumors. The chi-square test was used to compare the sex ratio between the patients with benign and malignant tumors.
Fifteen patients underwent RT surgery between 2009 and 2021. Demographics and clinical features of patients are in Table 1. Five patients had malignant, and 10 patients had benign tumors. The mean age of patients with malignant tumors was significantly higher than patients with benign mass (54.0 years vs. 36.1 years, P<0.05). In both groups with benign and malignant tumors, the percentage of female patients was more than male patients. There was no significant difference in sex ratio between patients with benign and malignant tumors (P>0.05).
Most patients were initially diagnosed by other specialists with nonspecific, vague, and chronic symptoms and were referred to a general surgeon. Among the patients, 73.3% had symptoms from a year ago and 13.3% were asymptomatic until diagnosis, which was detected incidentally by CT scan in the kidney stone workup (2 cases). Both asymptomatic patients had benign tumors, and patients with malignant tumors were all symptomatic. One malignant patient had a recurrence of renal cell carcinoma (RCC) tumor metastasis. The most common symptom in patients was pain around the tumor location, including low-back, pelvic, perineal, sacrum, or rectal pain (10 patients). Chronic constipation was the second most common symptom (7 patients). Other symptoms include urinary dysfunction (4 patients), perineal lump (2 patients), recurrent fistula (1 patient), fistula infection (1 patient), rectal bleeding (1 patient), vaginal bleeding (1 patient), and asymptomatic (2 patients).
On physical examination, 11 patients (73.3%) had a mass on the rectal examination; 6 patients had a mass on perineal examination, and 2 patients had a mass on vaginal examination. Mean tumor diameter in benign and malignant tumors were 9.6 and 8.2 cm, respectively, which did not show a significant difference (P>0.05).
All patients underwent a radiological examination before surgery, 4 patients underwent CT scan, and 11 patients underwent MRI. In addition, 6 patients underwent abdominal ultrasounds. Rectosigmoidoscopy was performed on 4 patients. RTs were reported solid in 8 patients, cystic in 4 patients, and heterogeneous in 3 patients. In the final interpretation, the radiologist reported 7 cases of suspected malignancy. After tumor resection, it was determined that 5 cases were malignant, and 2 were benign. In other words, in all 5 malignant patients, the radiologist had reported a malignant tumor before the pathology, but a radiological report of suspected malignancy was also reported in 2 benign patients.
The histopathology of resected RTs is given in Table 2. Histologically most common tumors were congenital tumors, 4 dermoid tumors, 4 chordoma tumors, 3 mature teratoma, 2 epidermoid tumors, 1 tailgut tumor, and 1 RCC metastasis tumor; to sum up, 5 tumors were malignant, and 10 tumors were benign.
A total of 3 patients underwent anterior approach surgery, 2 patients had tumors above S3, and 1 patient had tumor above and below S3. Also, 8 patients underwent posterior approach surgery, of which 7 patients had tumors below the S3 and 1 had a tumor at the top and bottom of S3. Table 3 shows surgical approaches to RTs and surgical outcomes for patients. A combined approach was made for 4 patients; 1 patient with a tumor above S3, 1 case with a tumor below the S3, and 2 cases with tumors above and below the S3. Of all 15 cases, 2 were operated laparoscopically (benign tumors).
The mean operation times in anterior, posterior, and combined surgery approaches were 196, 253, and 290 minutes, respectively. The differences were not statistically significant. This time was generally 261 minutes in open surgery and 190 minutes in laparoscopic surgery (P=0.005). The mean hospitalization times after surgery in the anterior, posterior, and combined approaches were 5.6, 5.2, and 7 days, respectively. This period was generally 6 days in open surgery and 4.5 days in laparoscopic surgery. The differences were not statistically significant.
Intraoperative complications rate in anterior, posterior, and combined approaches was 1, 4, and 2 cases, respectively. In 3 cases, the tumor cyst was ruptured during dissection, which had to be resected in fragments. One case encountered rectal perforation treated with primary repair and ileostomy diversion. Two cases experienced sacral bleeding, which was managed with local packing and was resolved during surgery. No acute bleeding after surgery was observed.
Postoperative complications were Clavien-Dindo classification grade I in 2 patients (13.3%), grade II in 3 patients (20.0%), and grade III in 1 patient (6.7%). Four cases had prolonged ileus for more than 5 days. Three cases encountered wound infection after surgery. Three patients had sexual dysfunction, 2 had urinary dysfunction, and 1 had a hematoma.
No biopsy or drainage of the cyst was performed on any of the patients before surgery. Complete resection with the meaning of complete resection of the tumor and free resection margins and removal of all lymph nodes involved in 14 cases was performed; only in 1 patient with teratoma, the removed margin of the tumor was reported positive in pathology. This patient was operated on with posterior approach surgery. During the 6-month follow-up of patients through history, clinical examination, and MRI, patients were evaluated for tumor recurrence or mortality. No mortality was seen after 30 days, and 2 cases expired after 6 months because of tumor recurrence (teratoma). In 13 other patients, no evidence of tumor recurrence was seen.
RTs are rare. The anatomy of retrorectal space is relatively complex as it contains multiple embryological residues [15]. RTs diagnosis can be made if the physician has good experience and a high degree of suspicion [16]. Radiography, including CT scan and MRI, can differentiate between tumors and make surgeons aware of tumor structure [11, 17]. Several individual case reports and a few large series evaluated RTs characteristics. Surgery is the main treatment and is mandatory in almost all cases [10]. We performed this study at Imam Khomeini Hospital as a tertiary center for over 12 years to report our experiences managing these tumors. In our series, most tumors (93%) were congenital, with female to male predominancy (2:1) in both benign and malignant tumors. Like our findings, Glasgow et al. [18] reported that most RTs cases were female, and the male sex had a significant correlation with malignancy risk.
Although CT scan can distinguish between solid and cystic lesions and determine tumor location and bony invasion [17, 19], nowadays, MRI provides more tissue contrast than CT scan that, determines soft-tissue planes, and evaluates the presence or absence of bony invasion, nerve and adjacent organs involvement or communication with dural space [20]. We used MRI or CT scan for all our patients; after pathological examination, we found that radiological examination with MRI or CT scan predicted malignancy with 100% sensitivity and 83% specificity; in the same way, Hopper et al. [3] in their study on 69 RTs patients, found that imaging can demonstrate malignant from benign tumors by 95% sensitivity and 64% specificity.
Some studies say that biopsy should perform only in unresectable tumors, and it is not recommended, especially if the tumor has a chance of infection or spreading [4, 21]. In our center, a multidisciplinary team decided on a surgery plan using patients’ MRI detail without presurgery biopsy. Due to the high sensitivity and specificity of MRI in previous studies and our experience, we think in most cases, the biopsy is not mandatory. On the contrary, Messick et al. [22] claimed that they used preoperative biopsy in 24 RTs patients without any evidence of biopsy site recurrence. And they profited from biopsy results to manage 3 patients without surgery.
Classic operative approaches to RTs are the abdominal or anterior approach, the transsacral or posterior approach, and the combined abdominal sacral approach [1]. Each approach has its particular use and considerations. The anterior approach is most commonly used when the tumor is located above the level of the 3rd sacral vertebra without sacral, sidewall, or visceral involvement [19]. The main advantage of this approach is the excellent view of pelvic structures, including large vessels and ureters [23, 24]. In our center, 3 patients underwent abdominal surgery, 2 of them had tumors above S3, and the third patient had a tumor above and below S3; in this case, due to the absence of sacrum or nerve roots and the surgeon’s opinion on the possibility of a successful exit from an anterior approach, this approach was selected. We have no evidence of tumor recurrence after anterior surgery with the least operation time comparing posterior and combined surgery, in the same way a review of 341 studies by Baek et al. [5] reported that the anterior approach had the least postoperative recurrence (P<0.05) but on the contrary, more operation time than posterior approach.
The posterior approach is utilized when the tumor is located below the level of the third sacral vertebra [3, 25]. Both transverse and longitudinal incisions can be made overlying the coccyx to facilitate excision and exposure to retrorectal space [26]. The retrorectal cavity can be reached as the anococcygeal ligament is cut. Bimanual rectal examination is an excellent way to establish a safe margin between the tumor and rectal wall [27]. In our study, 8 patients underwent a posterior approach; 7 had a tumor below S3, and 1 had a tumor above and below the S3, which was selected for sacrectomy and tumor resection through the posterior approach. We tried to decrease sacral resection as much as possible, preventing nerve injuries and patient complications. We saw that patients with the posterior approach experienced more complications than the anterior approach. We think it could be the result of partial sacrectomy causing nerve injuries and urinary or sexual dysfunction; some other studies mentioned these difficulties in managing pelvic vessels and nerves, as the most important disadvantage of the posterior approach [25, 28].
The combined approach has the advantages of both previously described techniques. It can be achieved when the patient is positioned in a lateral decubitus position or when the patient’s position changes during surgery consecutively from supine to jackknife (Kraske procedure) position [18, 29]. We performed the combined approach on 4 patients, 2 of them had tumors above and belove the S3. One of them had a tumor belove the S3, in this case, due to pelvic visceral involvement and the need for better access to the viscera through the abdominal approach and need to access to the tumor through the posterior approach, a combined approach was used. In another patient with a tumor above S3, a combined approach was used for posterior sacrectomy and abdominal tumor removal due to the big tumor size (11 cm) and sacrum involvement. Although it is not desired to open 2 surgical sites, because of the importance of complete resection of the tumor in conjunction with minimal damage, and inefficiency of abdominal or posterior approaches for some cases, we decided to choose a combined approach in these cases.
We observed that combined approach surgery had the highest operating time (P>0.05), length of hospital stays (P>0.05), and postoperative complications (P<0.05). We think these differences could be due to the larger and more advanced tumors operated by the combined approach and can also be due to the surgical method because, in the combined method, the patient is operated on from 2 areas, which may delay the patient’s recovery and increase the chance of surgical site infection and prolong the hospital stay. Contrary to us, Sakr et al. [10] in their review of 24 tailgut cysts reported that complications most frequently occurred in the posterior approach. And in the same way as us, they found that the combined approach had a longer operation time (P<0.001).
In a study by Mullaney et al. [8], a systematic review of 35 articles with a total number of 82 patients, the authors claimed that a minimally invasive approach for the resection of RT is a safe alternative associated with low morbidity and short length of stay. In the same way, we saw that laparoscopic surgery significantly reduced surgical time compared to open surgery (P<0.005). Laparoscopic surgery also reduced hospitalization time. Recently, Kwak and Ju [1] reported a huge tailgut cyst (7.8 cm) removed by the laparoscopic surgery with good visualization and hemostasis during surgery and no significant complication and desirable hospital stay, also some other studies reported difficulties in removing large tumors [30], but laparoscopic surgery advantages including reducing surgical trauma, neurological and vascular injuries in conjunction with same result comparing to open surgery, makes it a desirable method for wisely chosen cases.
Our study had limitations. Too much study time made it difficult to follow-up with patients closely; we had to exclude some cases from the survey due to the study’s retrospective nature and the lack of information. During a period of about 7 years in our center, laparoscopic surgery was not possible. Also, we did not have patients’ long-term survival details that could be informative.
In conclusion, RTs require complete surgical excision due to the possibility of malignancy and recurrence. None of the surgical methods has absolute precedence over the other. The choice of the surgical approach varies from patient to patient, and MRI findings play a crucial role in determining it. Choosing the most appropriate surgical method can reduce complications. Although the experience of laparoscopic surgery in RTs is limited, with the same efficiency as the open method, it can reduce the patient’s hospitalization time and pain.
Fig. 1.
The posterior approach. (A) Epidermoid cyst with no bone or adjacent organs involvement, sagittal magnetic resonance imaging (MRI) view. (B) Axial MRI view. (C) Tumor resection by anterior approach surgery.
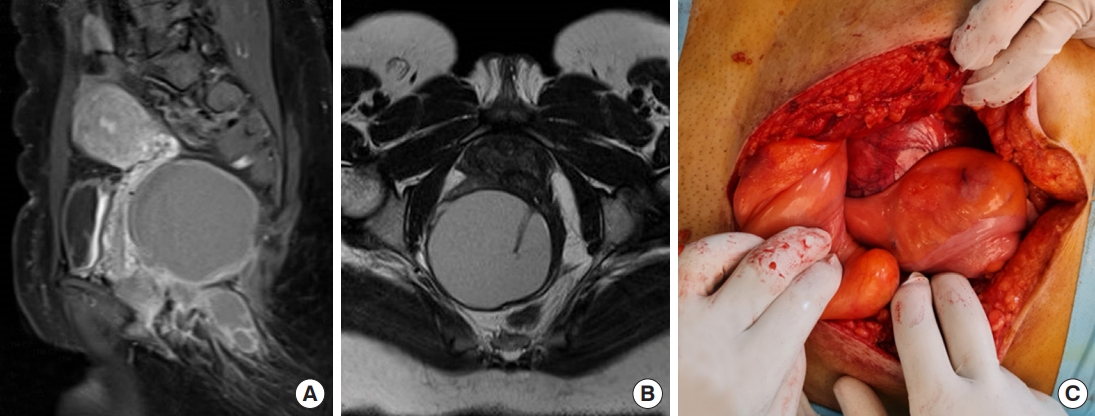
Fig. 2.
The abdominal approach. (A) Large retrorectal tumor with heterogeneous appearance. Magnetic resonance imaging (MRI) sagittal view. (B) Axial MRI view. (C) Posterior approach surgery with longitudinal postsacral incision (arrow) and total sacrectomy after tumor resection, the lateral incision (arrowhead) performed to cover dead space of posterior incision by gluteal flap.
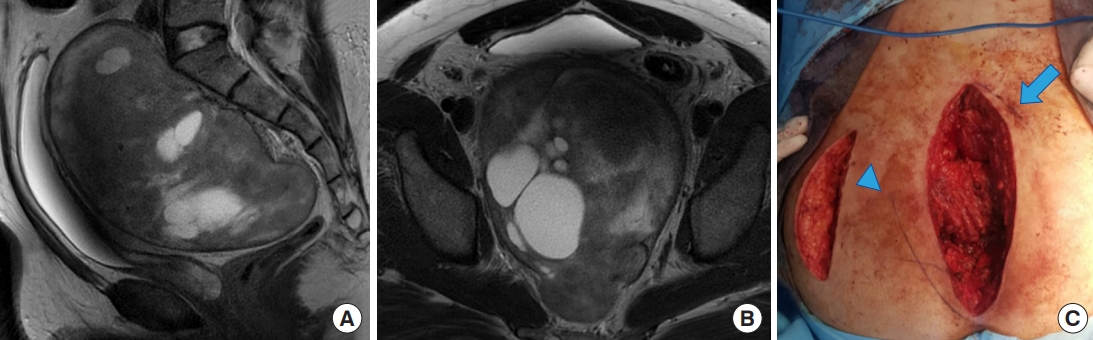
Fig. 3.
The combined approach. (A) Sagittal magnetic resonance imaging (MRI) of large chordoma with bone invasion, this tumor resected by a combined approach surgery with total sacral resection. (B) Coronal MRI view of the tumor. (C) En bloc resection of the tumor with sacral S3, S4, and lower part of S2.

Table 1.
Patients’ demographics and clinical characteristics (n=15)
Table 2.
Retrorectal tumors’ pathological results
| Variable | No. of cases |
|---|---|
| Congenital | |
| Dermoid cyst | 4 |
| Chordoma | 4 |
| Mature teratoma | 3 |
| Epidermoid cyst | 2 |
| Tailgut cyst | 1 |
| Miscellaneous | |
| Renal cell carcinoma metastasis | 1 |
Table 3.
Surgical approaches and outcomes
REFERENCES
1. Kwak HD, Ju JK. Laparoscopic resection of a huge retrorectal tumor. Ann Coloproctol 2020;36:54–7.




2. Jao SW, Beart RW Jr, Spencer RJ, Reiman HM, Ilstrup DM. Retrorectal tumors: Mayo Clinic experience, 1960-1979. Dis Colon Rectum 1985;28:644–52.


3. Hopper L, Eglinton TW, Wakeman C, Dobbs BR, Dixon L, Frizelle FA. Progress in the management of retrorectal tumours. Colorectal Dis 2016;18:410–7.


4. Toh JW, Morgan M. Management approach and surgical strategies for retrorectal tumours: a systematic review. Colorectal Dis 2016;18:337–50.


5. Baek SK, Hwang GS, Vinci A, Jafari MD, Jafari F, Moghadamyeghaneh Z, et al. Retrorectal tumors: a comprehensive literature review. World J Surg 2016;40:2001–15.



6. Wolpert A, Beer-Gabel M, Lifschitz O, Zbar AP. The management of presacral masses in the adult. Tech Coloproctol 2002;6:43–9.



7. Hobson KG, Ghaemmaghami V, Roe JP, Goodnight JE, Khatri VP. Tumors of the retrorectal space. Dis Colon Rectum 2005;48:1964–74.


8. Mullaney TG, Lightner AL, Johnston M, Kelley SR, Larson DW, Dozois EJ. A systematic review of minimally invasive surgery for retrorectal tumors. Tech Coloproctol 2018;22:255–63.



9. Yalav O, Topal U, Eray İC, Deveci MA, Gencel E, Rencuzogullari A. Retrorectal tumor: a single-center 10-years’ experience. Ann Surg Treat Res 2020;99:110–7.




10. Sakr A, Kim HS, Han YD, Cho MS, Hur H, Min BS, et al. Single-center experience of 24 cases of tailgut cyst. Ann Coloproctol 2019;35:268–74.




11. Woodfield JC, Chalmers AG, Phillips N, Sagar PM. Algorithms for the surgical management of retrorectal tumours. Br J Surg 2008;95:214–21.



12. Macafee DA, Sagar PM, El-Khoury T, Hyland R. Retrorectal tumours: optimization of surgical approach and outcome. Colorectal Dis 2012;14:1411–7.


13. Kye BH, Kim HJ, Cho HM, Chin HM, Kim JG. Clinicopathological features of retrorectal tumors in adults: 9 years of experience in a single institution. J Korean Surg Soc 2011;81:122–7.



16. Dahan H, Arrivé L, Wendum D, Docou le Pointe H, Djouhri H, Tubiana JM. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics 2001;21:575–84.


17. Johnson AR, Ros PR, Hjermstad BM. Tailgut cyst: diagnosis with CT and sonography. AJR Am J Roentgenol 1986;147:1309–11.


18. Glasgow SC, Birnbaum EH, Lowney JK, Fleshman JW, Kodner IJ, Mutch DG, et al. Retrorectal tumors: a diagnostic and therapeutic challenge. Dis Colon Rectum 2005;48:1581–7.


19. Boscà A, Pous S, Artés MJ, Gómez F, Granero Castro P, García-Granero E. Tumours of the retrorectal space: management and outcome of a heterogeneous group of diseases. Colorectal Dis 2012;14:1418-23.


20. Yang BL, Gu YF, Shao WJ, Chen HJ, Sun GD, Jin HY, et al. Retrorectal tumors in adults: magnetic resonance imaging findings. World J Gastroenterol 2010;16:5822–9.



21. Chéreau N, Lefevre JH, Meurette G, Mourra N, Shields C, Parc Y, et al. Surgical resection of retrorectal tumours in adults: long-term results in 47 patients. Colorectal Dis 2013;15:e476–82.


22. Messick CA, Hull T, Rosselli G, Kiran RP. Lesions originating within the retrorectal space: a diverse group requiring individualized evaluation and surgery. J Gastrointest Surg 2013;17:2143–52.



24. Bouts C, Van der Speeten K. A single center retrospective analysis of Kraske’s transsacral approach: a review. Surg Sci 2014;5:454–66.


25. Aranda-Narváez JM, González-Sánchez AJ, Montiel-Casado C, Sánchez-Pérez B, Jiménez-Mazure C, Valle-Carbajo M, et al. Posterior approach (Kraske procedure) for surgical treatment of presacral tumors. World J Gastrointest Surg 2012;4:126–30.



26. Althausen PL, Schneider PD, Bold RJ, Gupta MC, Goodnight JE Jr, Khatri VP. Multimodality management of a giant cell tumor arising in the proximal sacrum: case report. Spine (Phila Pa 1976) 2002;27:E361–5.


27. Freier DT, Stanley JC, Thompson NW. Retrorectal tumors in adults. Surg Gynecol Obstet 1971;132:681–6.

28. Buchs N, Taylor S, Roche B. The posterior approach for low retrorectal tumors in adults. Int J Colorectal Dis 2007;22:381–5.



- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 2,481 View
- 65 Download
- Related articles in ACP
-
Surgical Management of Sigmoid Volvulus: A Multicenter Observational Study2020 December;36(6)
Surgical Treatment of Recurrent Colorectal Cancer.2003 October;19(5)







