- Search
| Ann Coloproctol > Volume 39(5); 2023 > Article |
|
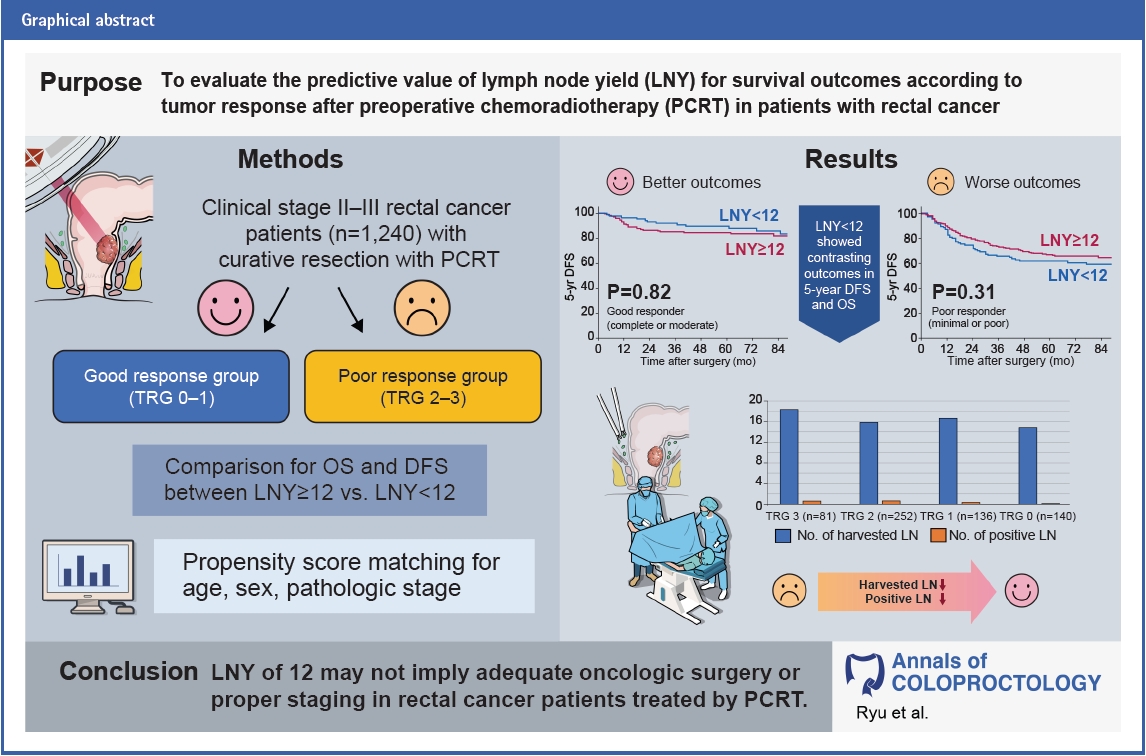
Abstract
Purpose
Methods
Results
Conclusion
Notes
Conflict of interest
In Ja Park is the Editor-in-Chief of Annals of Coloproctology, but was not involved in the review process of this article. No other potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: HSR, IJP; Data curation: all authors; Formal analysis: HSR, IJP; Investigation: all authors; Methodology: HSR, BKA; Project administration: IJP, SBL, JCK; Resources: YIK; Software: MSK, MYP; Supervision: IJP, SBL, JCK; Validation: IJK; Visualization: HSR, YIK; WritingŌĆōoriginal draft: HSR; WritingŌĆōreview & editing: all authors. All authors read and approved the final manuscript.
Fig.┬Ā1.
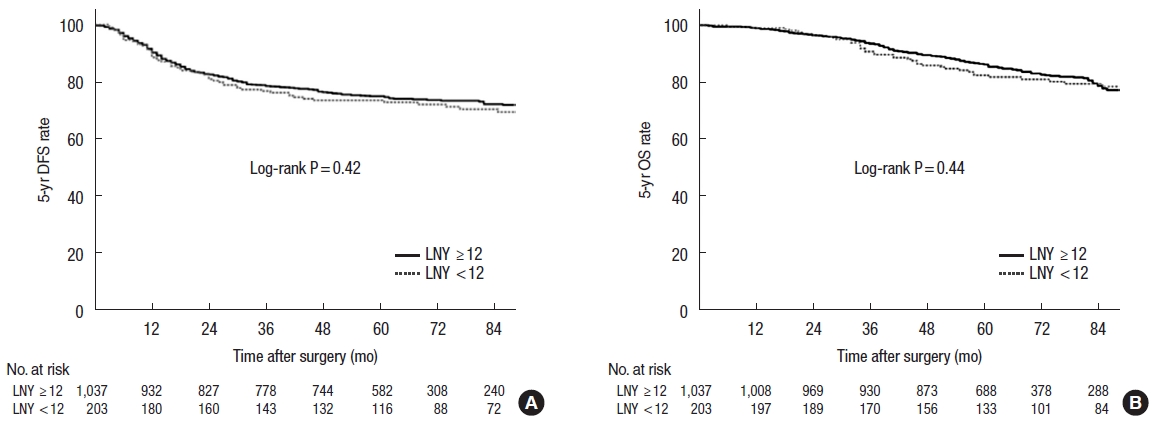
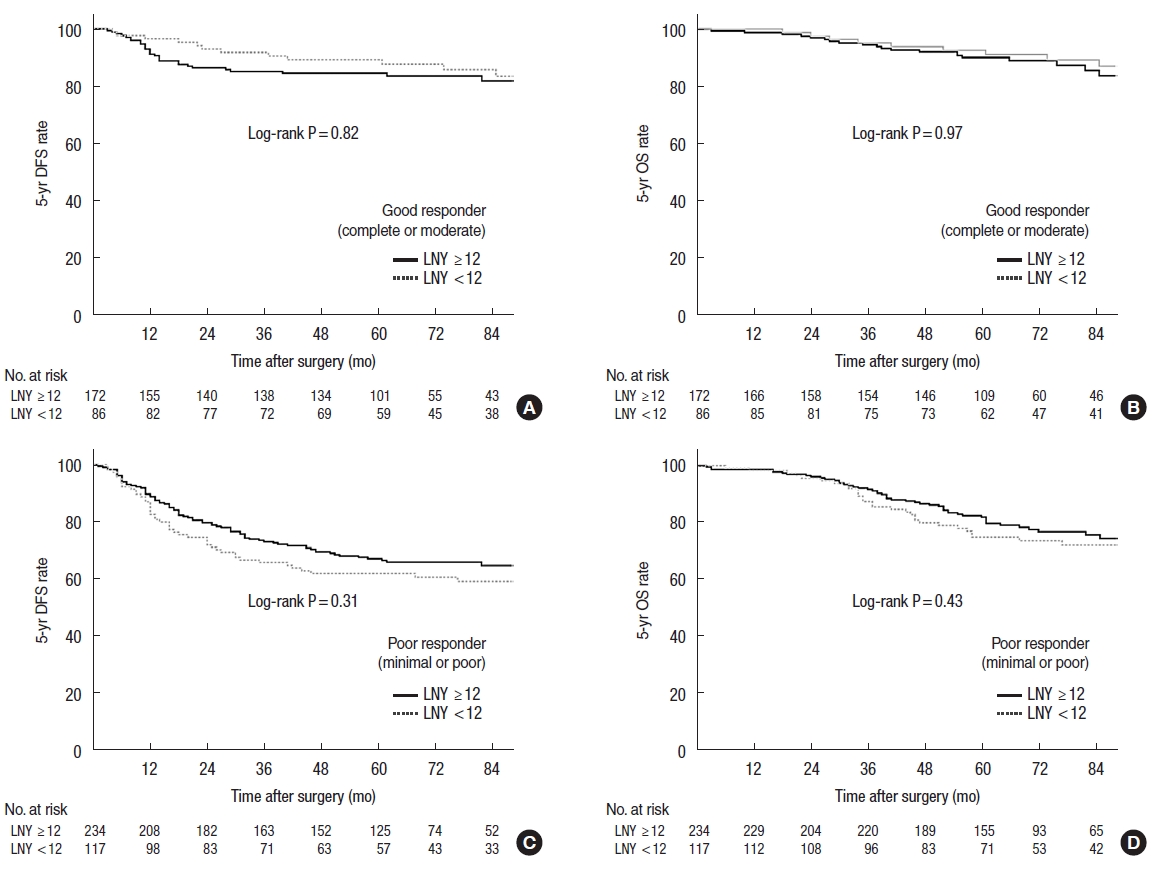
Fig.┬Ā2.
Table┬Ā1.
Table┬Ā2.
Table┬Ā3.
Table┬Ā4.
Table┬Ā5.
DFS, disease-free survival; OS overall survival; HR, hazard ratio; CI, confidence interval; WD, well-differentiated; MD, moderately differentiated; PD, poorly differenti┬Łated; UD, undifferentiated; LVI, lymphovascular invasion; PNI, perineural invasion; DRM, distal resection margin; CRM, circumferential margin; LNY, lymph node yield.
aThe variable listed first in the parenthesis is the reference category.
Table┬Ā6.
DFS, disease-free survival; OS overall survival; HR, hazard ratio; CI, confidence interval; WD, well-differentiated; MD, moderately differentiated; PD, poorly differenti┬Łated; UD, undifferentiated; LVI, lymphovascular invasion; PNI, perineural invasion; DRM, distal resection margin; CRM, circumferential margin; LNY, lymph node yield.
aThe variable listed first in the parenthesis is the reference category.
REFERENCES
- TOOLS
-
METRICS

- Related articles in ACP
-
Prognostic Significance of Tissue Leptin Expression in Colorectal Cancer Patients2015 December;31(6)