INTRODUCTION
Laparoscopic surgery for colorectal cancer has been widely accepted and adopted as a standard surgical option in recent years. Reported benefits of laparoscopic procedures include less pain, early recovery of the bowel function, early ambulation, and a shortened hospital stay [
1,
2]. However, even though laparoscopic surgery involves a less severe surgical incision and trauma to the abdominal wall, incisional hernia (IH) after laparoscopic colorectal surgery remains a frequent complication. IH can cause abdominal distension, discomfort, pain, cosmetic complaints, limitation of the activities of daily life and, in rare cases, strangulated hernia requiring an emergency operation.
The incidence of IH after laparoscopic colorectal surgery is reported to be 6% to 15% [
3–
9]. Even in cases with a limited, short incision, laparoscopic colorectal surgery was not significantly associated with a reduced incidence of IH in comparison to open surgery [
3,
10]. While several risk factors of IH after open colorectal surgery have been identified, those associated with laparoscopic surgery are still being debated. The clinical effect of less-invasive surgery with a laparoscopic approach should be considered and analyzed in a study examining the risk factors of IH.
The present study, therefore, investigated the risk factors for IH in cases of colorectal cancer after laparoscopic colorectal surgery.
RESULTS
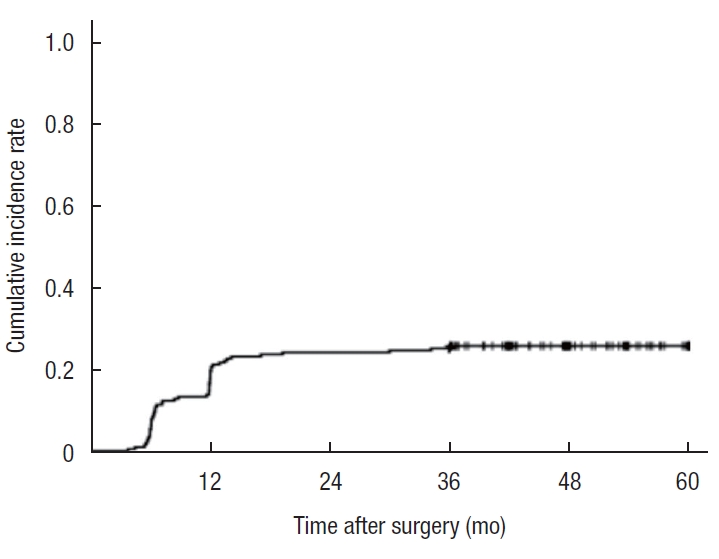
Among the 202 patients (113 men, 89 women) with colorectal cancer who underwent laparoscopic surgery, 52 patients (25.7%) were diagnosed with IH by CT. The cumulative incidence rate curve for IH is shown in
Fig. 2. Symptomatic IH was seen in 17 patients (8.4%). No perioperative mortality was encountered. The median observation period was 48 months. Most cases of IH developed within 2 years after the surgery (49 of 52, 94.2%).
Table 1 shows the results of the univariate analysis associated with IH. The univariate analysis showed that female sex (P=0.004), a high BMI (P<0.001), noncurrent smoking habit (P=0.043), low level of hemoglobin (P=0.035), high SFA (P<0.001), high VFA (P=0.006), low skeletal muscle area (P=0.001), long distance between the inner edges of the rectus abdominis muscle (P=0.001), long protrusion of the peritoneum at the umbilical site (P<0.001), and lymph node metastasis (P=0.007) were associated with IH according to Fisher exact test and Wilcoxon rank sum test. Regarding patient, tumor and operative factors, the age, serum level of albumin, preoperative therapy, tumor size, operative time, blood transfusion, and length of the skin incision were not correlated with IH. Wound infection was also not correlated with IH.
The multivariate logistic regression analysis revealed an older age (10-year increments: OR, 1.576; 95% CI, 1.027–2.419; P=0.037), lymph node metastasis (OR, 2.384; 95% CI, 1.132–5.018; P=0.022), and lengthy protrusion of the peritoneum at the umbilical site (10-mm increments: OR, 5.555; 95% CI, 3.058–10.091; P<0.001) were independent risk factors for IH (
Table 2).
DISCUSSION
In the present study, IH was diagnosed in 52 of the 202 patients (25.7%) by CT. This rate seemed to be relatively high compared with a previous report involving diagnoses by CT images (6%–15%) [
3–
9]. These reports included a relatively short follow-up period of at least 6 to 12 months [
4–
6]. The diagnosis of IH was made based on the discontinuity of the abdominal fascia at the umbilical surgical site with/without prolapse of any intra-abdominal organs by both surgeons and radiologists, and we included any-size IH in this study, as even small, asymptomatic IH has the potential to develop into symptomatic IH in the future. In addition, we considered it very important to make a strict diagnosis and analyze these small IHs as well in order to implement appropriate countermeasures in the future. Symptomatic IH was seen in 17 patients (8.4% in total patients, 32.7% in patient with IH diagnosed by CT). Reportedly, about 30% of IHs are missed on a physical examination [
14,
15]. Patients may be unaware of IH, perhaps due to severe obesity, the greater omentum entering the hernia or the hernia itself being small. In addition, it may be difficult to examine the hernia orifice in many obese patients because of the thickness of their subcutaneous fat. Therefore, appropriate evaluations in combination with the analysis of both CT findings and a physical examination are needed for the accurate evaluation of IH.
Several risk factors of IHs associated with open abdominal surgery have been identified [
16]. However, the incidence of IH was reportedly not markedly lower with laparoscopic colorectal surgery than with open surgery [
3,
10]. Laparotomy and laparoscopy differ in the degree of abdominal wall disruption induced, surgical invasiveness, and operative time. Thus, these procedures should be considered separately in terms of risk factors for IH. A number of risk factors for IH after laparoscopic colorectal surgery have been reported, including female sex, a high BMI, umbilical hernia, incision length, periumbilical midline incision, surgical site infection, and the VFA [
4,
5,
7,
8]. However, the incidence of IH does not seem to be markedly different in recent studies compared with earlier ones. Therefore, ongoing analyses and the implementation of countermeasures against risk factors of IH are needed.
In the present study, an older age was an independent risk factor for IH according to the multivariate analysis. Previous reports have also suggested that an older age is a well-known risk factor for IH after colorectal surgery, regardless of a laparoscopic or open procedure [
17,
18]. A poor nutritional status and/or the vulnerability of tissue with age was suggested to cause weakness of the abdominal wall [
17]. Given the current aging of society, opportunities to perform laparoscopic colorectal surgery in elderly patients are expected to increase. Therefore, the rate of complication of IH may also increase, becoming inevitable. Since the issue of age cannot be mitigated, identifying other age-related risk factors and adopting countermeasures will be necessary.
Our study demonstrated that an increased BMI, SFA, VFA, and decreased skeletal muscle area were significantly correlated with the incidence of IH in a univariate analysis. Even though those factors were not independent risk factors, there have been supportive previous reports regarding such obesity-related factors [
7,
8]. Yamamoto et al. [
7] and Fukuoka et al. [
8] reported that VFA of >100–110 cm
2 was an independent risk factor for IH, and the intra-abdominal pressure in obese patients is considered to depend more strongly on a direct mass effect from the intra-abdominal adipose tissue itself [
19]. These results indicated that even if the length of the incision is shorter after laparoscopic surgery than after open surgery, the presence of a larger amount of visceral fat would still influence the development of IH. In our study, the SFA was also related to the risk of IH in a univariate analysis. We speculated that this might be due to the difficulty of appropriately closing the abdominal wall because of the presence of excessive subcutaneous fat tissue and the reduced strength of the closure of the abdominal wall due to the innate weakness of the abdominal wall fascia. Therefore, preoperative assessment of the body composition and nutrition, and appropriate nutritional and dietary management by a nutritionist, which may help reduce the body fat mass while maintaining nutrition as preoperative preparatory activities at least while waiting for surgery, may be considered in the future. However, intentional prolonged intervention and rapid weight loss in a short period of time may cause the progression of cancer and decreased nutrition. Thus, this situation should be avoided.
Our study demonstrated a higher rate of female patients with IH than males. Previous reports have described conflicting findings, with different studies demonstrating female or male sex as an independent risk factor for IH after colorectal surgery [
3,
7]. These discrepancies regarding sex-specific influences on the risk of IH are considered to be due to the study population and possible sex-specific differences in wound healing due to differences in collagen metabolism between sexes [
7,
16]. Further studies with more cases should be conducted to resolve this issue.
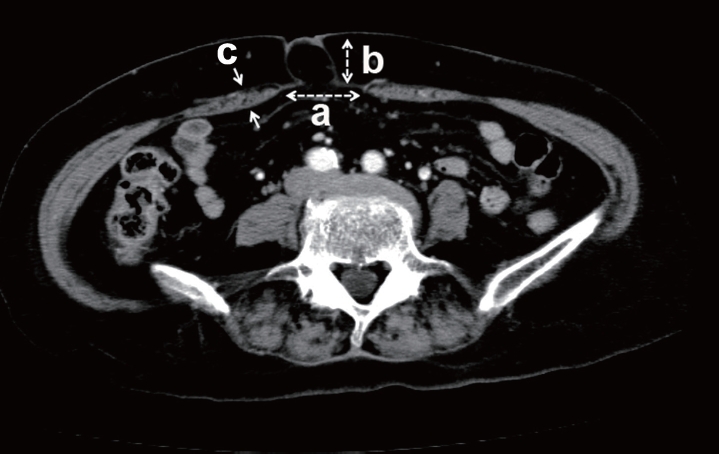
We also examined the distance between the inner edges of the rectus abdominis muscle and the distance of the protrusion of the umbilical peritoneum, as we sometimes experienced cases wherein umbilical hernia patients developed IH after their operation. In fact, Fukuoka et al. [
8] recently reported umbilical hernia as an independent risk factor for IH after laparoscopic colorectal surgery. We also tried to confirm the relationship between umbilical hernia and IH. However, the definition of umbilical hernia seemed to be ambiguous and depended on the discretion of the diagnostician. Therefore, we conducted a quantitative assessment of the umbilical site (
Fig. 1). A longer distance between the inner edges of the rectus abdominis muscle and the distance of protrusion of the peritoneum at the umbilical site were both associated with IH in our univariate analysis. Furthermore, a longer protrusion of the peritoneum at the umbilical site was the only independent risk factor for IH according to multivariate analysis. These results suggest that umbilical hernia may be a very important factor associated with the development of IH after abdominal surgery. The presence of an umbilical hernia might reflect the original tendency for an increased abdominal pressure due to a large amount of visceral fat, or it may reflect the original weakness of the fascia at the umbilical site.
In the present study, lymph node metastasis was frequently seen in patients with IH and was also an independent risk factor. There have been no previous reports regarding the association between lymph node metastasis and the incidence of IH. We also analyzed the influence of postoperative chemotherapy on the incidence of IH, because lymph node metastasis is related to a higher stage and the indication of postoperative chemotherapy. However, postoperative chemotherapy was not significantly associated with the occurrence of IH. Therefore, we speculate that it is possible that cases with lymph node metastasis may have also had a thicker mesentery due to their lymph node volume, which might have resulted in a larger incision of the abdominal wall during tumor extraction. Although no relationship was noted between the length of the surgical incision and IH in the univariate analysis, the skin incision may differ from the actual fascial incision because of its elasticity. The development of IH might be complex and related to a combination of the length of the fascial wound incision, the weakness of the fascia, and the thickness of the mesentery due to visceral fat and lymph node metastasis. In our study, the maximum tumor size and circumference rate of tumor were not correlated with the incidence of IH. Therefore, the tumor size might less markedly influence the size of the incision for tumor extraction than the thickness of the mesentery.
In addition to the factors we analyzed in this study, other factors may have contributed to the development of IH, including the patient's long-term nutritional status, underlying disease, and the state of control of coexisting diseases. Underlying diseases were observed and treated by the primary care physician in most of the patients during follow-up, and we lacked sufficient data for a combined analysis. Therefore, it would be interesting to examine these factors together in the future.
The present study was associated with some limitations, including its retrospective design and the fact that it was conducted at a single institution with a relatively small study population. Therefore, further studies will be needed in order to confirm the risk factors of IH after laparoscopic surgery for colorectal cancer.
In conclusion, our findings suggest that an older age, lymph node metastasis, and lengthy protrusion of the peritoneum at the umbilical site are risk factors for IH after laparoscopic surgery for colorectal cancer. An assessment using these factors before the operation and the implementation of countermeasures might help prevent IH. In addition, to prevent or minimize these problems, it might be interesting to reexamine the method of fascia suture, the specimen extraction site (e.g., a Pfannenstiel incision), and the use of a synthetic mesh with simultaneous colorectal surgery in the future.