- Search
|
|
Abstract
Purpose
Carcinoma arising from Crohn disease (CD) is rare, and there is no clear guidance on how to properly screen for at-risk patients and choose appropriate care. This study aimed to evaluate the clinicopathological characteristics, treatment, and oncologic outcomes of CD patients diagnosed with colorectal cancer (CRC).
Methods
Using medical records, we retrospectively enrolled a single-center cohort of 823 patients who underwent abdominal surgery for CD between January 2006 and December 2015. CD-associated CRC patients included those with adenocarcinoma, lymphoma, or neuroendocrine tumors of the colon and rectum.
Results
Nineteen patients (2.3%) underwent abdominal surgery to treat CD-associated CRC. The mean duration of CD in the CD-associated CRC group was significantly longer than that in the benign CD group (124.7┬▒77.7 months vs. 68.9┬▒60.2 months, P = 0.006). The CD-associated CRC group included a higher proportion of patients with a history of perianal disease (73.7% vs. 50.2%, P = 0.035) and colonic location (47.4% vs. 6.5%, P = 0.001). Among 19 CD-associated CRC patients, 17 (89.5%) were diagnosed with adenocarcinoma, and of the 17 cases, 15 (88.2%) were rectal adenocarcinoma. On multivariable analyses for developing CRC, only colonic location was a risk factor (relative risk, 7.735; 95% confidence interval, 2.862ŌĆō20.903; P = 0.001).
Crohn disease (CD) is a major form of inflammatory bowel disease (IBD) that can affect any site along the gastrointestinal tract and often necessitates surgical treatment [1]. Approximately 80% of patients with CD undergo at least 1 major intestinal surgery during their lifetime [2]. Indications for surgery among CD patients include fistula or abscess formation (50%), bowel stricture (30%), and bowel perforation (10%) [3]. Long-term medical treatment is required to prevent complications of CD, postoperatively. Rarely, long-standing CD can be complicated by malignancy [4].
The incidence of malignancy associated with CD is quite low, reportedly ranging between 0.3% and 0.8% [5]. Compared with patients from the West, Asian patients with malignant tumors associated with CD have been reported as more likely to have rectal cancer [6-8]. Korean patients with CD have been found to have higher rates of perianal fistula compared with Western patients, and most colorectal cancer (CRC) that develops from CD is associated with preexisting perianal fistula [9]. Additionally, mucinous cancer accounts for 56.3% of CD-associated malignancies, and 87.5% of cases are limited to the anorectal regions [10].
CRCs associated with perianal fistulas are relatively rare, and they tend to occur in patients who have had perianal fistulas for more than 10 years. Abdominoperineal resection (APR) is generally considered to be the surgical treatment of choice for carcinoma associated with perianal fistula. There are few articles with small series that describe sporadic cases of perianal cancer associated with CD fistulas [11, 12]. When malignancy occurs in association with CD and perianal fistula, delayed diagnosis, owing to the rareness of the association, can further complicate the scenario. Additionally, there is no clear guidance on how to properly screen for at-risk patients and select the appropriate management strategy. Besides, even when such patients are appropriately and promptly diagnosed, there is still no consensus as to whether they should receive standard treatments, such as preoperative chemotherapy followed by total mesorectal excision, as would administered for patients with sporadic rectal cancer.
This study aimed to evaluate the clinicopathological characteristics as well as the surgical and oncologic outcomes of patients with CD-associated CRC.
We retrospectively reviewed the medical records of 823 patients who underwent abdominal surgery for CD at Asan Medical Center, Seoul, Korea, between January 2006 and December 2015. Patients were included if they had undergone bowel resection, strictureplasty, stoma formation, or bypass surgery for CD during the study period and were 16 years of age or older at the time of surgery. Patients who underwent only stoma restoration were excluded.
CD-associated CRC was defined as malignancy of the colon and rectum that occurred in patients who were diagnosed with CD and had started medication to treat CD. The study included patients with colorectal adenocarcinoma, lymphoma, and neuroendocrine tumors. We excluded patients who were diagnosed with squamous cell carcinoma originating from the anus, cervical cancer, low-grade dysplasia, and metastatic carcinoma.
The following variables were captured during data collection: demographic and general information (age at diagnosis and operation; sex; duration of disease [duration between CD diagnosis and the last follow-up]; follow-up period; family history of CD; and history of smoking, perianal disease, abdominal surgery, or perianal surgery), clinical characteristics (Montreal classification and preoperative medications), operative findings (emergency operation, surgical approach, stoma formation, and operation time), operative outcomes (postoperative complications and hospital stay), histopathologic findings (tumor type, differentiation, anatomic association between perianal fistula and tumor, and stage), neoadjuvant or adjuvant treatment (chemotherapy or chemoradiotherapy [CRT]), and survival outcome.
Investigations for diagnosing and monitoring CD included small bowel series, computed tomography (CT) enterography, magnetic resonance imaging (MRI), and colonoscopy. Through these tests, the locations affected by CD could be confirmed, pathological features were evaluated, and, if necessary, surgical findings and postoperative histopathological findings were used for diagnosis [12].
When treating CD patients, a step-up approach is generally used in our institution, which means adding more potent therapies when the first-line or less toxic agents are not effective [13]. Gastroenterologists consult to specialized colorectal surgeons or the multidisciplinary team if a patient requires surgery.
The follow-up schedule for CRC usually proceeded as follows: clinical examinations, laboratory tests including carcinoembryonic agent, abdominopelvic CT every 6 months for the first 3 postoperative years, chest CT every 12 months, and colonoscopy or sigmoidoscopy after 1 postoperative year and then 2 or 3 years later. Patients suspected of recurrence underwent specific examinations (MRI, or positron emission tomography [PET]). Recurrence was diagnosed by radiological and histopathological methods using surgical resection or biopsy specimens [14].
Discrete variables, including demographic and preoperative characteristics, operative methods, and operative details were analyzed using the chi-square test to compare the CD-associated CRC group with the benign CD group. Continuous variables including, age at surgery, hospital stay, disease duration, and follow-up duration, were compared using unpaired Student t-test or the Mann-Whitney U-test as appropriate. The overall survival rate was examined using the Kaplan-Meier method and compared using the log-rank test. Multivariable analyses using binary logistic regression were used to assess the risks of developing CRC. Statistical significance was defined as P < 0.05, and all statistical analyses were performed using IBM SPSS Statistics for Windows, ver. 21 (IBM Corp., Armonk, NY, USA).
A total of 823 patients were finally included in this study. Nineteen patients (2.3%) underwent abdominal surgery for CD-associated CRC, and 804 patients (97.7%) underwent abdominal surgery for benign CD during the study period. The mean age at the time of surgery among patients in the CD-associated CRC group was significantly older than that among patients in benign group (39.2┬▒11.3 years vs. 31.5┬▒9.9 years, P= 0.002). The mean duration of CD in the CD-associated CRC group was significantly longer than that in the benign CD group (124.7┬▒77.7 months vs. 68.9┬▒60.2 months, P = 0.006). The CD-associated CRC group had significantly higher proportions of patients with a history of perianal disease (78.9% vs. 50.2%, P= 0.035) and abdominal surgery (57.9% vs. 33.8%, P= 0.001). Compared with the benign CD group, the CD-associated CRC group had a significantly higher proportion of patients with non-stricturing and non-penetrating (B1) CD (42.1% vs. 4.7%, P = 0.001), and a significantly lower proportion of patients with penetrating (B3) CD (26.3% vs. 60.6%, P= 0.001), according to the Montreal classification. At the time of surgery, the CD-associated CRC group had a significantly lower proportion of patients with ileal disease (5.3% vs. 42.5%, P= 0.001) and a significantly higher proportion of patients with colonic disease (47.4% vs. 6.5%, P= 0.001), compared with the benign CD group. Variables that did not differ significantly between these 2 groups included age at diagnosis, sex, follow-up duration, family history of CD, history of smoking, history of perianal surgery, age at diagnosis by the Montreal classification, and preoperative medications (Table 1).
Of the 19 patients with CD-associated CRC, 16 (84.2%) underwent stoma formation procedures (including either permanent or diverting stomas), while 127 benign CD patients (15.8%) underwent stoma formation procedures (P= 0.001). The mean operating time among patients in the CD-associated CRC group was significantly longer than that among patients in the benign CD group (250┬▒123 minutes vs. 158┬▒61 minutes, P = 0.001). The rates of emergency operations, surgical approach, postoperative complications (including infectious and intra-abdominal complications), as well as the mean postoperative hospital stay duration were not significantly different between the 2 groups (Table 2).
Within the CD-associated CRC group, 17 patients (89.5%) were diagnosed with adenocarcinoma, 1 patient (5.3%) was diagnosed with a neuroendocrine carcinoma and the other patient (5.3%) was diagnosed with plasmablastic lymphoma. Seven patients (36.8%) were already diagnosed with stage IV cancer at the time of diagnosis. Among the 17 patients with colorectal adenocarcinoma, 15 (88.2%) had rectal adenocarcinoma, and 2 (11.8%) had colonic adenocarcinoma (Fig. 1). Ten cases (58.8%) of colorectal adenocarcinoma were related to an anatomical association between perianal fistula and tumor. Among the 17 patients with colorectal adenocarcinoma, 3 (17.6%) had poorly-differentiated carcinoma, 5 (29.4%) had mucinous differentiation, and 3 (17.6 %) had signet ring cell carcinoma (unfavorable histology). At the time of surgery, 14 patients (73.7%) had been diagnosed with CD more than 5 years earlier, and 8 patients (42.1%) had been diagnosed with CD more than 10 years earlier (Table 3).
At the time of diagnosis, 5 patients (26.3%) whose tumors were inoperable underwent only stoma formation without radical surgery. Eight patients (42.1%) underwent APR, 3 patients (17.6%) underwent total colectomy or total proctocolectomy (TPC) due to severe colitis, and 3 patients (17.6%) underwent segmental resection and anastomosis (right colectomy or low anterior resection). Although more than half of the cases were associated with unfavorable histology, 8 patients (42.1%) received preoperative or postoperative radiotherapy. A patient was finally diagnosed with pathologic complete remission (pCR) after receiving preoperative CRT, followed by APR (Table 3).
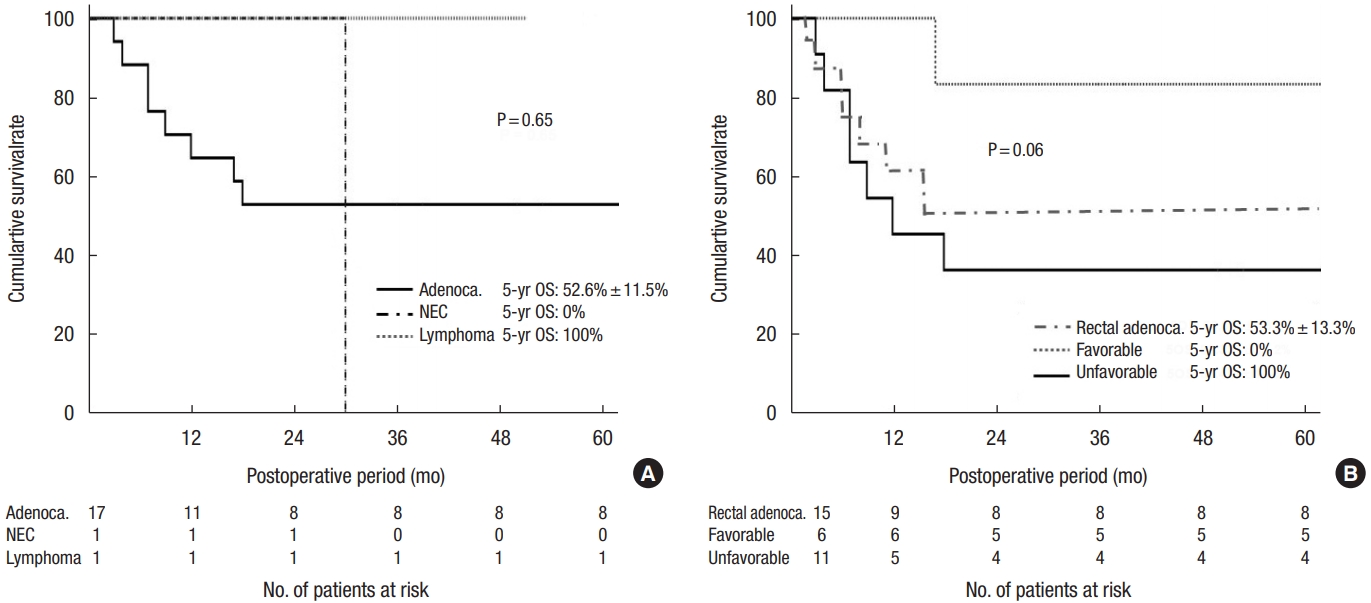
The 5-year overall survival rates of the 17 patients with colorectal adenocarcinoma and 15 patients with rectal adenocarcinoma were 52.6%┬▒11.5% and 53.3%┬▒13.0%, respectively. One patient with stage IV neuroendocrine carcinoma died within 30 postoperative months, and 1 patient with stage I lymphoma was alive with recurrence after 51 months (Figs. 1, 2). One patient with stage IVA colon cancer died within 18 postoperative months, and 1 patient with stage IIA colon cancer was alive without a recurrence after 123 months (Fig. 1). The 5-year overall survival rate of the 11 patients with unfavorable adenocarcinoma was relatively lower than that of the 6 patients with favorable adenocarcinoma without a significance (36.4%┬▒14.5% vs. 83.3%┬▒15.2%, P= 0.06) (Fig. 2, Supplementary Table 1). Among the 17 patients with adenocarcinoma, 7 patients (41.2%) remained alive without recurrence at the latest follow-up date. Of 8 patients with regional stages of rectal adenocarcinoma (pCR, stage I, and II), 6 (75.0%) were alive with (n= 4) or without recurrence (n= 2).
On additional multivariable analyses for developing CRC, only the L3 classification (CD colitis) was revealed as a risk factor of developing CRC (relative risk, 7.735; 95% confidence interval, 2.862ŌĆō20.903; P= 0.001).
The present study showed a CD-associated CRC prevalence of 2.3% among patients who underwent abdominal surgery for CD. Unsurprisingly, this prevalence was higher than that of the general CD population, as the present study included patients who had abdominal surgery. Although there has been some controversy regarding the increased risk of developing cancer in association with CD since the first report of CD-associated CRC in 1948 [15], a population-based study demonstrated an increased risk of CRC, and a recent meta-analysis also showed CD patients have a 2.4-fold overall increased risk of developing CRC [16-18]. A study reported that, among CD patients, the cumulative incidence of CRC was 0.3% at 5 years, 1.6% at 15 years, and 2.4% at 25 years, and a cross-sectional comparison revealed a 1.9% increased risk of CD-associated CRC, which is similar to the results of the present study [19]. From the literature, this relatively high prevalence supports that inflammation plays an important role in the development of CRC, especially considering that CD patients who have undergone surgery are usually affected by more active disease and longer duration [20, 21].
The present study demonstrated the association between the anatomic location of the CD involvement and the development of CRC. The CD-associated CRC group had a significantly higher rate of perianal fistula history, and more than half of the CD-associated CRC had an anatomical association between perianal fistula and tumor. There have been some reports of fistula-associated CRC in CD patients, and those reports also suggested a possible association between perianal fistula and CRC [9, 11, 22]. A review article showed the correlation between colitis (inflammation) and cancer risk and discussed how inflammation might contribute to CRC pathogenesis (inflammation-dysplasia-cancer model) [23]. Similarly, the present study showed that the L3 classification (CD colitis) was revealed as a risk factor for developing CD-associated CRC. Smoking and long-standing disease duration have also been found to be independent risk factors for developing CRC among CD patients [24, 25]. Because smoking rate of both groups in the present is low, it seems difficult to determine whether smoking is a risk factor for CRC. Although the mean disease duration of the CD-associated CRC group was significantly longer than that of the benign CD group, the present study did not show that disease duration was a risk factor for developing CRC. However, considering that more than 40% of CD-associated patients have been CD for more than 10 years, the present study might suggest that physicians should be alerted to the possibility of CRC when they observe perianal fistula or CD colitis with long-standing CD duration.
Surgery for CD-associated CRC is complicated by the conflicting objectives of minimal bowel resection and appropriate tumor resection. For CD-associated CRC, American Society of Colon and Rectal Surgery clinical practice guidelines insist on TPC for multiplicity and to reduce the risk of metachronous CRC [26]. Although the extent of resection required can depend on the location of the malignancy, it is notable that multifocal dysplasia is observed in more than one-third of specimens from patients who have undergone colectomy [27]. Besides, 14% to 40% of the patients who undergo segmental resection for cancer of the large intestine develop metachronous colorectal cancers, which supports the call for TPC for CD-associated CRC [27, 28]. However, only 17% of the patients in the present study underwent total colectomy or TPC, partly because 40% of the patients had inoperable disease at the time of diagnosis; surgeon preferences also played a role in the low proportion of total colectomy and TPC. The present study also found no metachronous CRC or multiplicity in the specimens, so further research is needed, as the follow-up duration was relatively short.
Although a high rate of stage IV CRC was observed in the present study, patients with early stage (pCR, stage I, and stage II) and favorable histology had a relatively good 5-year overall survival rate. This finding highlights the importance of early detection in terms of facilitating good oncologic outcomes. However, it is difficult to diagnose fistula-associated CRC because it is difficult to distinguish malignancy from CD colitis in perianal fistulas. In general, symptoms of CD-associated CRC are non-specific, and the sensitivity of the imaging test (CT, MRI, and PET) is relatively low owing to inflammation, and this can lead to delayed diagnosis [9, 29, 30]. Patients with a long-term history of perianal fistula have been found to have high cancer stages at the time of surgery [11, 31]. For managing perianal fistulas, early detection of CRC, and managing CD-associated CRC, it is ideal to use a multidisciplinary team approach including gastroenterologists, colorectal surgeons, oncologists, and radiologists.
The prognosis of CD-associated CRC patients after surgery is largely determined by the CRC stage after surgery [32]. The present study showed that prognosis, histologic type, and stage were related, it was encouraging that there was a patient who went into pCR after receiving preoperative CRT. In terms of quality of life, the rate of stoma formation was more than 80% in the present study, and this was related to the anatomical association between perianal fistula and tumor and poor outcomes associated with pouch surgery in CD [33]. From these results, it is apparent that a multimodality approach incorporating early detection and appropriate surgery and CRT may produce good functional and oncologic results even in patients with CD-associated CRC.
Because the present study was retrospective and cross-sectional in design, we could not control for clinicopathological characteristics. However, the study evaluated both CD-associated CRC and benign CD characteristics, including demographics, surgical methods, pathologic findings, and postoperative and oncologic outcomes, using a relatively large patient cohort. Another limitation of this study was the lack of analysis of risk factors for developing CRC in association with CD due to the small number of CD-associated CRC patients. Nevertheless, the present study was meaningful, as it included a larger number of patients than other retrospective studies, and it clarified the prevalence of CD-associated CRC among patients who underwent abdominal surgery.
In conclusion, the diagnosis of colorectal malignancy is rare among CD patients who have undergone abdominal surgery and the L3 classification (CD colitis) was a risk factor of developing CRC. Rectal adenocarcinoma accounted for most of the CRC, and the subgroup with unfavorable histology had poor overall survival rate.
SUPPLEMENTARY MATERIALS
Supplementary materials for this study are presented online (available at https://doi.org/10.3393/ac.2020.11.02).
Supplementary Table 1. Clinicopathological characteristics and survival of the patients with Crohn disease (CD)-associated colorectal adenocarcinoma according to histologic type
Fig.┬Ā1.
Classification and survival outcomes of the patients with Crohn disease (CD)-associated colorectal cancer (CRC). NEC, neuroendocrine carcinoma; GI, gastrointestinal; NED, no evidence of disease; OS, overall survival.
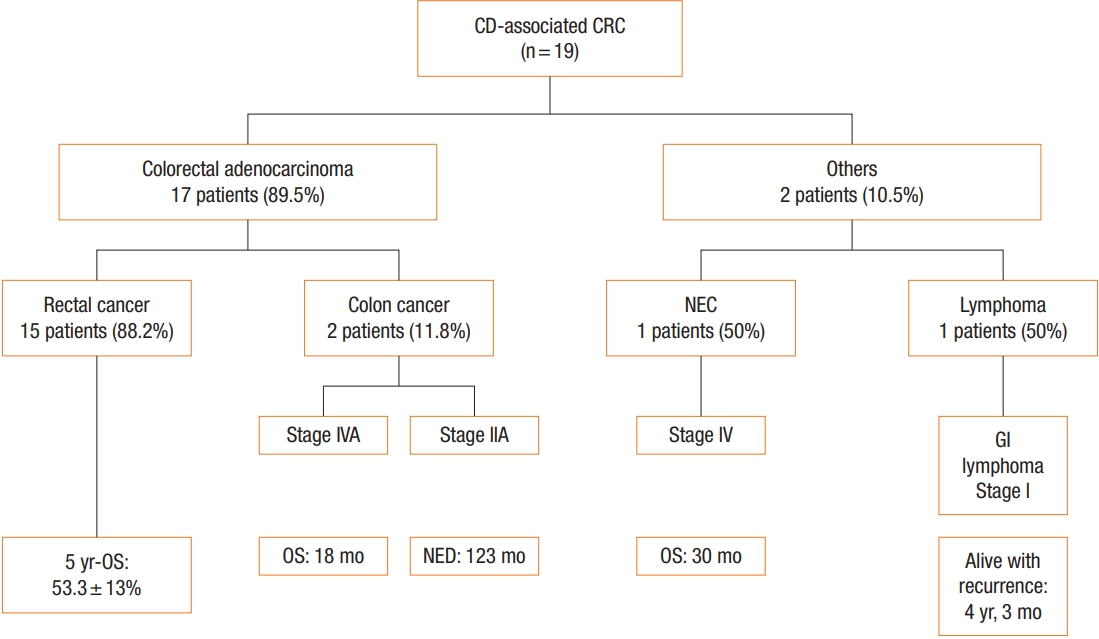
Fig.┬Ā2.
Overall survival (OS) outcome according to tumor type (A) (Adeno., adenocarcinoma; NEC, neuroendocrine carcinoma; Lymphoma) and histologic type (B) (favorable, well-differentiated and moderately-differentiated; unfavorable, poorly-differentiated, mucinous, and signetring cell).
Table┬Ā1.
Clinical characteristics of the patients with CD-associated CRC and benign CD
| Variable | CD-associated CRC | Benign CD | P-value |
|---|---|---|---|
| No. of patients | 19 | 804 | |
| Age (yr) | |||
| ŌĆāAt diagnosis | 29.7 ┬▒ 10.2 (23ŌĆō36) | 26.1 ┬▒ 10.0 (19ŌĆō31) | 0.150 |
| ŌĆāAt operation | 39.2 ┬▒ 11.3 (32ŌĆō49) | 31.5 ┬▒ 9.9 (24ŌĆō37) | 0.009* |
| Sex, female:male | 7 (36.8):12 (63.2) | 233 (29.0):571 (71.0) | 0.450 |
| Duration of disease (mo) | 124.7 ┬▒ 77.7 (66ŌĆō172) | 68.9 ┬▒ 60.2 (14ŌĆō109) | 0.006* |
| Follow-up period (mo) | 81.7 ┬▒ 39.1 (48ŌĆō107) | 85.1 ┬▒ 39.1 (59ŌĆō111) | 0.710 |
| Family history of Crohn disease, yes | 0 (0) | 24 (3.0) | |
| History of smoking | 0.630 | ||
| ŌĆāNone | 11 (57.9) | 528 (65.7) | |
| ŌĆāEx-smoker | 5 (26.3) | 199 (24.8) | |
| ŌĆāCurrent smoker | 3 (15.8) | 77 (9.6) | |
| History of perianal diseasea | 0.035* | ||
| ŌĆāYes | 15 (78.9) | 404 (50.2) | |
| ŌĆāNo | 4 (21.1) | 400 (49.8) | |
| History of abdominal surgery | 0.001* | ||
| ŌĆāYes | 11 (57.9) | 272 (33.8) | |
| ŌĆāNo | 8 (42.1) | 532 (66.2) | |
| History of perianal surgery | 0.960 | ||
| ŌĆāYes | 12 (63.2) | 332 (41.3) | |
| ŌĆāNo | 7 (36.1) | 471 (58.7) | |
| Montreal classification | |||
| ŌĆāAge at diagnosis (yr) | 0.990 | ||
| ŌĆāŌĆāŌēż 16 | 2 (10.5) | 89 (11.1) | |
| ŌĆāŌĆā> 16, Ōēż 40 | 15 (78.9) | 639 (79.4) | |
| ŌĆāŌĆā> 40 | 2 (10.5) | 76 (9.5) | |
| ŌĆāBehavior at operation | 0.001* | ||
| ŌĆāŌĆāNon-stricturing, non-penetrating (B1) | 8 (42.1) | 38 (4.7) | |
| ŌĆāŌĆāStricturing (B2) | 6 (31.6) | 279 (34.7) | |
| ŌĆāŌĆāPenetrating (B3) | 5 (26.3) | 487 (60.6) | |
| Montreal classification location at operation | 0.001* | ||
| ŌĆāIleum (L1) | 1 (5.3) | 343 (42.7) | |
| ŌĆāColon (L2) | 9 (47.4) | 52 (6.5) | |
| ŌĆāIleocolon (L3) | 9 (47.4) | 408 (50.7) | |
| Medication | |||
| ŌĆāNo medication or 5-ASA only | 5 (26.3) | 340 (42.5) | 0.239 |
| ŌĆāImmunomodulator | 8 (42.1) | 253 (31.5) | 0.330 |
| ŌĆāanti-TNF-╬▒ | 1 (5.3) | 48 (6.0) | 0.990 |
| ŌĆāSteroid | 2 (10.5) | 41 (5.1) | 0.260 |
| ŌĆāImmunomodulator with anti-TNF-╬▒ | 3 (15.8) | 57 (7.1) | 0.070 |
| ŌĆāImmunomodulator with steroid | 0 (0) | 43 (5.3) | 0.618 |
| ŌĆāSteroid with anti-TNF-╬▒ | 0 (0) | 8 (1.0) | 0.990 |
| ŌĆāAll combinations | 0 (0) | 12 (1.5) | 0.990 |
Table┬Ā2.
Operative finding and postoperative outcome of the patients with CD-associated CRC and benign CD
| Variable | CD-associated CRC (n = 19) | Benign CD (n = 804) | P-value |
|---|---|---|---|
| Emergency operation | 1 (5.3) | 70 (8.7) | 0.990 |
| Surgical approach | 0.780 | ||
| ŌĆāOpen | 16 (84.2) | 638 (79.4) | |
| ŌĆāLaparoscopy | 3 (15.8) | 166 (20.6) | |
| Stoma formation | 0.001* | ||
| ŌĆāYes | 16 (84.2) | 127 (15.8) | |
| ŌĆāNo | 3 (16.8) | 677 (84.2) | |
| Operation time (min) | 250 ┬▒ 123 (177ŌĆō286) | 158 ┬▒ 61 (113ŌĆō187) | 0.001* |
| Postoperative complication | 0.920 | ||
| ŌĆāYes | 6 (31.6) | 202 (25.1) | |
| ŌĆāNo | 13 (68.4) | 602 (74.9) | |
| Infectious complication | 0.710 | ||
| ŌĆāYes | 5 (26.3) | 152 (18.9) | |
| ŌĆāNo | 14 (73.7) | 652 (81.1) | |
| Intraabdominal complication | 0.650 | ||
| ŌĆāYes | 3 (15.8) | 77 (9.6) | |
| ŌĆāNo | 16 (84.2) | 727 (90.4) | |
| Hospital stay after operation (day) | 16.5 ┬▒ 27.6 (5ŌĆō21) | 13.1 ┬▒ 15.0 (7ŌĆō13) | 0.340 |
Table┬Ā3.
Clinicopathological characteristics and survival of the patients with CD-associated colorectal cancer
Values are presented as number only, number (%), or mean┬▒standard deviation (interquartile range).
CD, Crohn disease; NEC, neuroendocrine carcinoma; anti-TNF-╬▒, anti-tumor necrosis factor ╬▒; WD, well-differentiated; MD, moderately-differentiated; PD, poorly-differentiated; SRC, signet-ring cell carcinoma; APR, abdominoperineal resection; TC, total colectomy; TPC, total proctocolectomy; RHC, right hemicolectomy; uLAR, ultra-low anterior resection; CAA, coloanal anastomosis; pCR, pathologic complete remission; CCRT, chemoradiotherapy; CTx, chemotherapy.
REFERENCES
1. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal CrohnŌĆÖs disease. Br J Surg 2000;87:1697ŌĆō701.


3. Lewis RT, Maron DJ. Efficacy and complications of surgery for CrohnŌĆÖs disease. Gastroenterol Hepatol (N Y) 2010;6:587ŌĆō96.


4. Cahill C, Gordon PH, Petrucci A, Boutros M. Small bowel adenocarcinoma and CrohnŌĆÖs disease: any further ahead than 50 years ago? World J Gastroenterol 2014;20:11486ŌĆō95.



5. Laukoetter MG, Mennigen R, Hannig CM, Osada N, Rijcken E, Vowinkel T, et al. Intestinal cancer risk in CrohnŌĆÖs disease: a metaanalysis. J Gastrointest Surg 2011;15:576ŌĆō83.


7. Gillen CD, Andrews HA, Prior P, Allan RN. CrohnŌĆÖs disease and colorectal cancer. Gut 1994;35:651ŌĆō5.



8. Kim J, Lee HS, Park SH, Yang SK, Ye BD, Yang DH, et al. Pathologic features of colorectal carcinomas associated with CrohnŌĆÖs disease in Korean population. Pathol Res Pract 2017;213:250ŌĆō5.


9. Ogawa H, Haneda S, Shibata C, Miura K, Nagao M, Ohnuma S, et al. Adenocarcinoma associated with perianal fistulas in CrohnŌĆÖs disease. Anticancer Res 2013;33:685ŌĆō9.

10. Lee KM, Lee JM. CrohnŌĆÖs disease in Korea: past, present, and future. Korean J Intern Med 2014;29:558ŌĆō70.



11. Iesalnieks I, Gaertner WB, Glass H, Strauch U, Hipp M, Agha A, et al. Fistula-associated anal adenocarcinoma in CrohnŌĆÖs disease. Inflamm Bowel Dis 2010;16:1643ŌĆō8.


12. Connell WR, Sheffield JP, Kamm MA, Ritchie JK, Hawley PR, Lennard-Jones JE. Lower gastrointestinal malignancy in CrohnŌĆÖs disease. Gut 1994;35:347ŌĆō52.



13. Sobala A, Herbst F, Novacek G, Vogelsang H. Colorectal carcinoma and preceding fistula in CrohnŌĆÖs disease. J Crohns Colitis 2010;4:189ŌĆō93.


14. Yang KM, Yu CS, Lee JL, Kim CW, Yoon YS, Park IJ, et al. Risk factors for postoperative recurrence after primary bowel resection in patients with CrohnŌĆÖs disease. World J Gastroenterol 2017;23:7016ŌĆō24.



15. Warren S, Sommers SC. Cicatrizing enteritis as a pathologic entity; analysis of 120 cases. Am J Pathol 1948;24:475ŌĆō501.


16. Persson PG, Karl├®n P, Bernell O, Leijonmarck CE, Brostr├Čm O, Ahlbom A, et al. CrohnŌĆÖs disease and cancer: a population-based cohort study. Gastroenterology 1994;107:1675ŌĆō9.


17. Greenstein AJ, Sachar DB, Smith H, Janowitz HD, Aufses AH Jr. A comparison of cancer risk in CrohnŌĆÖs disease and ulcerative colitis. Cancer 1981;48:2742ŌĆō5.


18. von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with CrohnŌĆÖs disease. Dis Colon Rectum 2007;50:839ŌĆō55.


19. Jess T, Loftus EV Jr, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology 2006;130:1039ŌĆō46.


20. Scaringi S, Di Martino C, Zambonin D, Fazi M, Canonico G, Leo F, et al. Colorectal cancer and CrohnŌĆÖs colitis: clinical implications from 313 surgical patients. World J Surg 2013;37:902ŌĆō10.


21. Maykel JA, Hagerman G, Mellgren AF, Li SY, Alavi K, Baxter NN, et al. CrohnŌĆÖs colitis: the incidence of dysplasia and adenocarcinoma in surgical patients. Dis Colon Rectum 2006;49:950ŌĆō7.


22. Shwaartz C, Munger JA, Deliz JR, Bornstein JE, Gorfine SR, Chessin DB, et al. Fistula-associated anorectal cancer in the setting of CrohnŌĆÖs disease. Dis Colon Rectum 2016;59:1168ŌĆō73.


23. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287:G7ŌĆō17.


24. Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2009;7:682ŌĆō8.


25. Sj├Čdahl RI, Myrelid P, S├Čderholm JD. Anal and rectal cancer in CrohnŌĆÖs disease. Colorectal Dis 2003;5:490ŌĆō5.


26. Strong S, Steele SR, Boutrous M, Bordineau L, Chun J, Stewart DB, et al. Clinical practice guideline for the surgical management of crohnŌĆÖs disease. Dis Colon Rectum 2015;58:1021ŌĆō36.


27. Kiran RP, Nisar PJ, Goldblum JR, Fazio VW, Remzi FH, Shen B, et al. Dysplasia associated with CrohnŌĆÖs colitis: segmental colectomy or more extended resection? Ann Surg 2012;256:221ŌĆō6.

28. Maser EA, Sachar DB, Kruse D, Harpaz N, Ullman T, Bauer JJ. High rates of metachronous colon cancer or dysplasia after segmental resection or subtotal colectomy in CrohnŌĆÖs colitis. Inflamm Bowel Dis 2013;19:1827ŌĆō32.


29. Devon KM, Brown CJ, Burnstein M, McLeod RS. Cancer of the anus complicating perianal CrohnŌĆÖs disease. Dis Colon Rectum 2009;52:211ŌĆō6.


30. Basseri RJ, Basseri B, Vassilaki ME, Melmed GY, Ippoliti A, Vasiliauskas EA, et al. Colorectal cancer screening and surveillance in CrohnŌĆÖs colitis. J Crohns Colitis 2012;6:824ŌĆō9.


31. Thomas M, Bienkowski R, Vandermeer TJ, Trostle D, Cagir B. Malignant transformation in perianal fistulas of CrohnŌĆÖs disease: a systematic review of literature. J Gastrointest Surg 2010;14:66ŌĆō73.


- TOOLS








